Microbial Communities Involved in Methane, Sulfur, and Nitrogen Cycling in the Sediments of the Barents Sea
- PMID: 34835487
- PMCID: PMC8625253
- DOI: 10.3390/microorganisms9112362
Microbial Communities Involved in Methane, Sulfur, and Nitrogen Cycling in the Sediments of the Barents Sea
Abstract
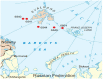
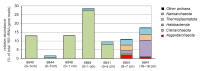
A combination of physicochemical and radiotracer analysis, high-throughput sequencing of the 16S rRNA, and particulate methane monooxygenase subunit A (pmoA) genes was used to link a microbial community profile with methane, sulfur, and nitrogen cycling processes. The objects of study were surface sediments sampled at five stations in the northern part of the Barents Sea. The methane content in the upper layers (0-5 cm) ranged from 0.2 to 2.4 µM and increased with depth (16-19 cm) to 9.5 µM. The rate of methane oxidation in the oxic upper layers varied from 2 to 23 nmol CH4 L-1 day-1 and decreased to 0.3 nmol L-1 day-1 in the anoxic zone at a depth of 16-19 cm. Sulfate reduction rates were much higher, from 0.3 to 2.8 µmol L-1 day-1. In the surface sediments, ammonia-oxidizing Nitrosopumilaceae were abundant; the subsequent oxidation of nitrite to nitrate can be carried out by Nitrospira sp. Aerobic methane oxidation could be performed by uncultured deep-sea cluster 3 of gamma-proteobacterial methanotrophs. Undetectable low levels of methanogenesis were consistent with a near complete absence of methanogens. Anaerobic methane oxidation in the deeper sediments was likely performed by ANME-2a-2b and ANME-2c archaea in consortium with sulfate-reducing Desulfobacterota. Sulfide can be oxidized by nitrate-reducing Sulfurovum sp. Thus, the sulfur cycle was linked with the anaerobic oxidation of methane and the nitrogen cycle, which included the oxidation of ammonium to nitrate in the oxic zone and denitrification coupled to the oxidation of sulfide in the deeper sediments. Methane concentrations and rates of microbial biogeochemical processes in sediments in the northern part of the Barents Sea were noticeably higher than in oligotrophic areas of the Arctic Ocean, indicating that an increase in methane concentration significantly activates microbial processes.
Keywords: Barents Sea; arctic; marine sediments; methane cycle; microbial communities; nitrogen cycle; sulfur cycle.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- James R.H., Bousquet P., Bussmann I., Haeckel M., Kipfer R., Leifer I., Niemann H., Ostrovsky I., Piskozub J., Rehder G., et al. Effects of climate change on methane emissions from seafloor sediments in the Arctic Ocean: A review. Limnol. Oceanogr. 2016;61:283–299. doi: 10.1002/lno.10307. - DOI
-
- Hunter S.J., Goldobin D.S., Haywood A.M., Ridgewell A., Rees J.G. Sensitivity of the global submarine hydrate inventory to scenarios of future climate change. Earth Planet. Sci. Lett. 2013:105–115. doi: 10.1016/j.epsl.2013.02.017. - DOI
-
- Reagan M.T., Moridis G.J., Elliott S.M., Maltrud M. Contribution of oceanic gas hydrate dissociation to the formation of Arctic Ocean methane plumes. J. Geophys. Res. 2011;116 doi: 10.1029/2011JC007189. - DOI
-
- Reagan M.T., Moridis G.J. Large-scale simulation of methane hydrate dissociation along the West Spitsbergen Margin. Geophys. Res. Lett. 2009;36 doi: 10.1029/2009GL041332. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

