Use of Sedatives and Neuromuscular-Blocking Agents in Mechanically Ventilated Patients with COVID-19 ARDS
- PMID: 34835518
- PMCID: PMC8624865
- DOI: 10.3390/microorganisms9112393
Use of Sedatives and Neuromuscular-Blocking Agents in Mechanically Ventilated Patients with COVID-19 ARDS
Abstract
Objectives: To assess differences in the use of analgesics, sedatives and neuromuscular-blocking agents (NMBA) in patients with acute respiratory distress syndrome (ARDS) due to COVID-19 or other conditions.
Methods: Retrospective observational cohort study, single-center tertiary Intensive Care Unit. COVID-19 patients with ARDS (March-May 2020) and non-COVID ARDS patients (2017-2020) on mechanical ventilation and receiving sedation for at least 48 h.
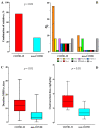
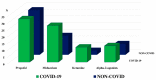
Results: A total of 39 patients met the inclusion criteria in each group, with similar demographics at baseline. COVID-19 patients had a longer duration of MV (median 22 (IQRs 16-29) vs. 9 (6-18) days; p < 0.01), of sedatives administration (18 (11-22) vs. 5 (4-9) days; p < 0.01) and NMBA therapy (12 (9-16) vs. 3 (2-7) days; p < 0.01). During the first 7 days of sedation, compared to non-COVID patients, COVID patients received more frequently a combination of multiple sedative drugs (76.9% vs. 28.2%; p < 0.01) and a higher NMBA regimen (cisatracurium: 3.0 (2.1-3.7) vs. 1.3 (0.9-1.9) mg/kg/day; p < 0.01).
Conclusions: The duration and consumption of sedatives and NMBA was significantly increased in patients with COVID-19 related ARDS than in non-COVID ARDS. Different sedation strategies and protocols might be needed in COVID-19 patients with ARDS, with potential implications on long-term complications and drugs availability.
Keywords: COVID-19; acute respiratory distress syndrome; neuromuscular-blocking agents; sedation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Kim L., Garg S., O’Halloran A., Whitaker M., Pham H., Anderson E.J., Armistead I., Bennett N.M., Billing L., Como-Sabetti K., et al. Risk Factors for Intensive Care Unit Admission and In-hospital Mortality Among Hospitalized Adults Identified through the US Coronavirus Disease 2019 (COVID-19)-Associated Hospitalization Surveillance Network (COVID-NET) Clin. Infect. Dis. 2020;72:e206–e214. doi: 10.1093/cid/ciaa1012. - DOI - PMC - PubMed
-
- Di Castelnuovo A., Bonaccio M., Costanzo S., Gialluisi A., Antinori A., Berselli N., Blandi L., Bruno R., Cauda R., Guaraldi G., et al. Cardiovascular risk factors and in-hospital mortality in 3,894 patients with COVID-19: Survival analysis and machine learning-based findings from the multicentre Italian CORIST Study. Nutr. Metab. Cardiovasc. Dis. 2020;30:1899–1913. doi: 10.1016/j.numecd.2020.07.031. - DOI - PMC - PubMed
-
- Taccone F.S., Van Goethem N., De Pauw R., Wittebole X., Blot K., Van Oyen H., Lernout T., Montourcy M., Meyfroidt G., Van Beckhoven D. The role of organizational characteristics on the outcome of COVID-19 patients admitted to the ICU in Belgium. Lancet Reg. Health Eur. 2021;2:100019. doi: 10.1016/j.lanepe.2020.100019. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

