New Insights into the Host-Pathogen Interaction of Mycoplasma gallisepticum and Avian Metapneumovirus in Tracheal Organ Cultures of Chicken
- PMID: 34835532
- PMCID: PMC8618481
- DOI: 10.3390/microorganisms9112407
New Insights into the Host-Pathogen Interaction of Mycoplasma gallisepticum and Avian Metapneumovirus in Tracheal Organ Cultures of Chicken
Abstract
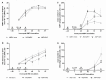
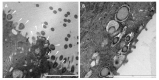
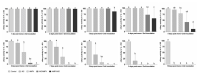
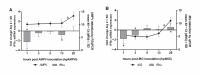
Respiratory pathogens are a health threat for poultry. Co-infections lead to the exacerbation of clinical symptoms and lesions. Mycoplasma gallisepticum (M. gallispeticum) and Avian Metapneumovirus (AMPV) are two avian respiratory pathogens that co-circulate worldwide. The knowledge about the host-pathogen interaction of M. gallispeticum and AMPV in the chicken respiratory tract is limited. We aimed to investigate how co-infections affect the pathogenesis of the respiratory disease and whether the order of invading pathogens leads to changes in host-pathogen interaction. We used chicken tracheal organ cultures (TOC) to investigate pathogen invasion and replication, lesion development, and selected innate immune responses, such as interferon (IFN) α, inducible nitric oxide synthase (iNOS) and IFNλ mRNA expression levels. We performed mono-inoculations (AMPV or M. gallispeticum) or dual-inoculations in two orders with a 24-h interval between the first and second pathogen. Dual-inoculations compared to mono-inoculations resulted in more severe host reactions. Pre-infection with AMPV followed by M. gallispeticum resulted in prolonged viral replication, more significant innate immune responses, and lesions (p < 0.05). AMPV as the secondary pathogen impaired the bacterial attachment process. Consequently, the M. gallispeticum replication was delayed, the innate immune response was less pronounced, and lesions appeared later. Our results suggest a competing process in co-infections and offer new insights in disease processes.
Keywords: Mycoplasma gallisepticum; TOC; avian metapneumovirus; co-infection; innate immunity; interferon.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Felice V., Lupini C., Mescolini G., Silveira F., Guerrini A., Catelli E., Di Francesco A. Molecular detection and characterization of Mycoplasma gallisepticum and Mycoplasma synoviae strains in backyard poultry in Italy. Poult. Sci. 2020;99:719–724. doi: 10.1016/j.psj.2019.12.020. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

