Facile Synthesis of N-Doped Graphene Quantum Dots as Novel Transfection Agents for mRNA and pDNA
- PMID: 34835580
- PMCID: PMC8620666
- DOI: 10.3390/nano11112816
Facile Synthesis of N-Doped Graphene Quantum Dots as Novel Transfection Agents for mRNA and pDNA
Abstract
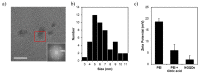
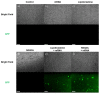
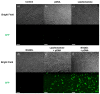
In the wake of the coronavirus disease 2019 (COVID-19) pandemic, global pharmaceutical companies have developed vaccines for the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Some have adopted lipid nanoparticles (LNPs) or viral vectors to deliver the genes associated with the spike protein of SARS-CoV-2 for vaccination. This strategy of vaccination by delivering genes to express viral proteins has been successfully applied to the mRNA vaccines for COVID-19, and is also applicable to gene therapy. However, conventional transfection agents such as LNPs and viral vectors are not yet sufficient to satisfy the levels of safety, stability, and efficiency required for the clinical applications of gene therapy. In this study, we synthesized N-doped graphene quantum dots (NGQDs) for the transfection of various genes, including messenger ribonucleic acids (mRNAs) and plasmid deoxyribonucleic acids (pDNAs). The positively charged NGQDs successfully formed electrostatic complexes with negatively charged mRNAs and pDNAs, and resulted in the efficient delivery and transfection of the genes into target cells. The transfection efficiency of NGQDs is found to be comparable to that of commercially available LNPs. Considering their outstanding stability even at room temperature as well as their low toxicity, NGQDs are expected to be novel universal gene delivery platforms that can outperform LNPs and viral vectors.
Keywords: gene delivery; graphene quantum dots; mRNA; pDNA; transfection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

