Tween® Preserves Enzyme Activity and Stability in PLGA Nanoparticles
- PMID: 34835710
- PMCID: PMC8625811
- DOI: 10.3390/nano11112946
Tween® Preserves Enzyme Activity and Stability in PLGA Nanoparticles
Abstract
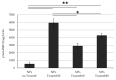
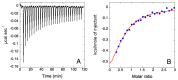
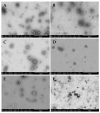
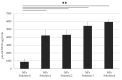
Enzymes, as natural and potentially long-term treatment options, have become one of the most sought-after pharmaceutical molecules to be delivered with nanoparticles (NPs); however, their instability during formulation often leads to underwhelming results. Various molecules, including the Tween® polysorbate series, have demonstrated enzyme activity protection but are often used uncontrolled without optimization. Here, poly(lactic-co-glycolic) acid (PLGA) NPs loaded with β-glucosidase (β-Glu) solutions containing Tween® 20, 60, or 80 were compared. Mixing the enzyme with Tween® pre-formulation had no effect on particle size or physical characteristics, but increased the amount of enzyme loaded. More importantly, NPs made with Tween® 20:enzyme solutions maintained significantly higher enzyme activity. Therefore, Tween® 20:enzyme solutions ranging from 60:1 to 2419:1 mol:mol were further analyzed. Isothermal titration calorimetry analysis demonstrated low affinity and unquantifiable binding between Tween® 20 and β-Glu. Incorporating these solutions in NPs showed no effect on size, zeta potential, or morphology. The amount of enzyme and Tween® 20 in the NPs was constant for all samples, but a trend towards higher activity with higher molar rapports of Tween® 20:β-Glu was observed. Finally, a burst release from NPs in the first hour with Tween®:β-Glu solutions was the same as free enzyme, but the enzyme remained active longer in solution. These results highlight the importance of stabilizers during NP formulation and how optimizing their use to stabilize an enzyme can help researchers design more efficient and effective enzyme loaded NPs.
Keywords: Tween® stabilization; enzyme delivery; enzyme stabilization; nanomedicine; polymeric nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Synthesis, characterization, and evaluation of paclitaxel loaded in six-arm star-shaped poly(lactic-co-glycolic acid).Int J Nanomedicine. 2013;8:4315-26. doi: 10.2147/IJN.S51629. Epub 2013 Nov 7. Int J Nanomedicine. 2013. PMID: 24235829 Free PMC article.
-
Enzyme Stability in Nanoparticle Preparations Part 1: Bovine Serum Albumin Improves Enzyme Function.Molecules. 2020 Oct 9;25(20):4593. doi: 10.3390/molecules25204593. Molecules. 2020. PMID: 33050145 Free PMC article.
-
Poly(lactide)-vitamin E derivative/montmorillonite nanoparticle formulations for the oral delivery of Docetaxel.Biomaterials. 2009 Jul;30(19):3297-306. doi: 10.1016/j.biomaterials.2009.02.045. Epub 2009 Mar 19. Biomaterials. 2009. PMID: 19299012
-
[Formulation and process optimization of doxorubicin-loaded PLGA nanoparticles and its in vitro release].Yao Xue Xue Bao. 2013 May;48(5):759-66. Yao Xue Xue Bao. 2013. PMID: 23888702 Chinese.
-
Calcium hydroxide-loaded PLGA biodegradable nanoparticles as an intracanal medicament.Int Endod J. 2021 Nov;54(11):2086-2098. doi: 10.1111/iej.13603. Epub 2021 Sep 1. Int Endod J. 2021. PMID: 34355406
Cited by
-
Optimization of an Injectable Hydrogel Depot System for the Controlled Release of Retinal-Targeted Hybrid Nanoparticles.Pharmaceutics. 2022 Dec 21;15(1):25. doi: 10.3390/pharmaceutics15010025. Pharmaceutics. 2022. PMID: 36678654 Free PMC article.
-
Tunneling Nanotubes: A New Target for Nanomedicine?Int J Mol Sci. 2022 Feb 17;23(4):2237. doi: 10.3390/ijms23042237. Int J Mol Sci. 2022. PMID: 35216348 Free PMC article. Review.
-
Quantitative comparison of the protein corona of nanoparticles with different matrices.Int J Pharm X. 2022 Oct 21;4:100136. doi: 10.1016/j.ijpx.2022.100136. eCollection 2022 Dec. Int J Pharm X. 2022. PMID: 36304137 Free PMC article.
-
Applications of the ROS-Responsive Thioketal Linker for the Production of Smart Nanomedicines.Polymers (Basel). 2022 Feb 11;14(4):687. doi: 10.3390/polym14040687. Polymers (Basel). 2022. PMID: 35215600 Free PMC article. Review.
-
"Combo" Multi-Target Pharmacological Therapy and New Formulations to Reduce Inflammation and Improve Endogenous Remyelination in Traumatic Spinal Cord Injury.Cells. 2023 May 6;12(9):1331. doi: 10.3390/cells12091331. Cells. 2023. PMID: 37174731 Free PMC article.
References
-
- Rigon L., Salvalaio M., Pederzoli F., Legnini E., Duskey J.T., D’Avanzo F., De Filippis C., Ruozi B., Marin O., Vandelli M.A., et al. Targeting Brain Disease in MPSII: Preclinical Evaluation of IDS-Loaded PLGA Nanoparticles. Int. J. Mol. Sci. 2019;20:2014. doi: 10.3390/ijms20082014. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

