The Evolution and Future of Targeted Cancer Therapy: From Nanoparticles, Oncolytic Viruses, and Oncolytic Bacteria to the Treatment of Solid Tumors
- PMID: 34835785
- PMCID: PMC8623458
- DOI: 10.3390/nano11113018
The Evolution and Future of Targeted Cancer Therapy: From Nanoparticles, Oncolytic Viruses, and Oncolytic Bacteria to the Treatment of Solid Tumors
Abstract
While many classes of chemotherapeutic agents exist to treat solid tumors, few can generate a lasting response without substantial off-target toxicity despite significant scientific advancements and investments. In this review, the paths of development for nanoparticles, oncolytic viruses, and oncolytic bacteria over the last 20 years of research towards clinical translation and acceptance as novel cancer therapeutics are compared. Novel nanoparticle, oncolytic virus, and oncolytic bacteria therapies all start with a common goal of accomplishing therapeutic drug activity or delivery to a specific site while avoiding off-target effects, with overlapping methodology between all three modalities. Indeed, the degree of overlap is substantial enough that breakthroughs in one therapeutic could have considerable implications on the progression of the other two. Each oncotherapeutic modality has accomplished clinical translation, successfully overcoming the potential pitfalls promising therapeutics face. However, once studies enter clinical trials, the data all but disappears, leaving pre-clinical researchers largely in the dark. Overall, the creativity, flexibility, and innovation of these modalities for solid tumor treatments are greatly encouraging, and usher in a new age of pharmaceutical development.
Keywords: clinical trials; exosomes; nanoparticles; oncolytic bacteria; oncolytic viruses; solid tumors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

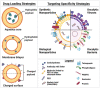



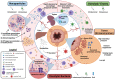


Similar articles
-
Hacking the Immune Response to Solid Tumors: Harnessing the Anti-Cancer Capacities of Oncolytic Bacteria.Pharmaceutics. 2023 Jul 21;15(7):2004. doi: 10.3390/pharmaceutics15072004. Pharmaceutics. 2023. PMID: 37514190 Free PMC article. Review.
-
The next frontier of oncotherapy: accomplishing clinical translation of oncolytic bacteria through genetic engineering.Future Microbiol. 2021 Mar;16(5):341-368. doi: 10.2217/fmb-2020-0245. Epub 2021 Mar 23. Future Microbiol. 2021. PMID: 33754804 Free PMC article. Review.
-
Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy.Eur J Pharm Biopharm. 2015 Jun;93:52-79. doi: 10.1016/j.ejpb.2015.03.018. Epub 2015 Mar 23. Eur J Pharm Biopharm. 2015. PMID: 25813885 Review.
-
Oncolytic Virotherapy for Cancer: Clinical Experience.Biomedicines. 2021 Apr 13;9(4):419. doi: 10.3390/biomedicines9040419. Biomedicines. 2021. PMID: 33924556 Free PMC article. Review.
-
Oncolytic viruses for cancer immunotherapy.J Hematol Oncol. 2020 Jun 29;13(1):84. doi: 10.1186/s13045-020-00922-1. J Hematol Oncol. 2020. PMID: 32600470 Free PMC article. Review.
Cited by
-
Hacking the Immune Response to Solid Tumors: Harnessing the Anti-Cancer Capacities of Oncolytic Bacteria.Pharmaceutics. 2023 Jul 21;15(7):2004. doi: 10.3390/pharmaceutics15072004. Pharmaceutics. 2023. PMID: 37514190 Free PMC article. Review.
-
An intravenous pancreatic cancer therapeutic: Characterization of CRISPR/Cas9n-modified Clostridium novyi-Non Toxic.PLoS One. 2023 Nov 14;18(11):e0289183. doi: 10.1371/journal.pone.0289183. eCollection 2023. PLoS One. 2023. PMID: 37963142 Free PMC article.
-
Nanomaterial-assisted oncolytic bacteria in solid tumor diagnosis and therapeutics.Bioeng Transl Med. 2024 Apr 17;9(4):e10672. doi: 10.1002/btm2.10672. eCollection 2024 Jul. Bioeng Transl Med. 2024. PMID: 39036084 Free PMC article. Review.
-
Nanotechnology for Pediatric Retinoblastoma Therapy.Pharmaceuticals (Basel). 2022 Aug 31;15(9):1087. doi: 10.3390/ph15091087. Pharmaceuticals (Basel). 2022. PMID: 36145308 Free PMC article. Review.
-
Exploring the role of oral bacteria in oral cancer: a narrative review.Discov Oncol. 2025 Feb 26;16(1):242. doi: 10.1007/s12672-025-01998-2. Discov Oncol. 2025. PMID: 40009328 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources

