Charge-Modulated Synthesis of Highly Stable Iron Oxide Nanoparticles for In Vitro and In Vivo Toxicity Evaluation
- PMID: 34835832
- PMCID: PMC8624538
- DOI: 10.3390/nano11113068
Charge-Modulated Synthesis of Highly Stable Iron Oxide Nanoparticles for In Vitro and In Vivo Toxicity Evaluation
Abstract
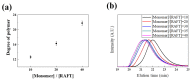
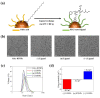
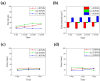
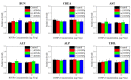
The surface charge of iron oxide nanoparticles (IONPs) plays a critical role in the interactions between nanoparticles and biological components, which significantly affects their toxicity in vitro and in vivo. In this study, we synthesized three differently charged IONPs (negative, neutral, and positive) based on catechol-derived dopamine, polyethylene glycol, carboxylic acid, and amine groups, via reversible addition-fragmentation chain transfer-mediated polymerization (RAFT polymerization) and ligand exchange. The zeta potentials of the negative, neutral, and positive IONPs were -39, -0.6, and +32 mV, respectively, and all three IONPs showed long-term colloidal stability for three months in an aqueous solution without agglomeration. The cytotoxicity of the IONPs was studied by analyzing cell viability and morphological alteration in three human cell lines, A549, Huh-7, and SH-SY5Y. Neither IONP caused significant cellular damage in any of the three cell lines. Furthermore, the IONPs showed no acute toxicity in BALB/c mice, in hematological and histological analyses. These results indicate that our charged IONPs, having high colloidal stability and biocompatibility, are viable for bio-applications.
Keywords: PEG ligands; biocompatibility; colloidal stability PEG ligands; iron oxide nanoparticles; toxicity.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Serga V., Burve R., Maiorov M., Krumina A., Skaudžius R., Zarkov A., Kareiva A., Popov A.I. Impact of gadolinium on the structure and magnetic properties of nanocrystalline powders of iron oxides produced by the extraction-pyrolytic method. Materials. 2020;13:4147. doi: 10.3390/ma13184147. - DOI - PMC - PubMed
-
- Kahil H., Faramawy A., El-Sayed H., Abdel-Sattar A. Magnetic properties and SAR for gadolinium-doped iron oxide nanoparticles prepared by hydrothermal method. Crystals. 2021;11:1153. doi: 10.3390/cryst11101153. - DOI
-
- Qiao J., Wang T., Zheng K., Zhou E., Shen C., Jia A., Zhang Q. Magnetically reusable Fe3O4@NC@Pt catalyst for selective reduction of nitroarenes. Catalysts. 2021;11:1219. doi: 10.3390/catal11101219. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

