Metabolomic Response of Early-Stage Wheat (Triticum aestivum) to Surfactant-Aided Foliar Application of Copper Hydroxide and Molybdenum Trioxide Nanoparticles
- PMID: 34835836
- PMCID: PMC8622224
- DOI: 10.3390/nano11113073
Metabolomic Response of Early-Stage Wheat (Triticum aestivum) to Surfactant-Aided Foliar Application of Copper Hydroxide and Molybdenum Trioxide Nanoparticles
Abstract
Surfactants are commonly used in foliar applications to enhance interactions of active ingredients with plant leaves. We employed metabolomics to understand the effects of TritonTM X-100 surfactant (SA) and nanomaterials (NMs) on wheat (Triticum aestivum) at the molecular level. Leaves of three-week-old wheat seedlings were exposed to deionized water (DI), surfactant solution (SA), NMs-surfactant suspensions (Cu(OH)2 NMs and MoO3 NMs), and ionic-surfactant solutions (Cu IONs and Mo IONs). Wheat leaves and roots were evaluated via physiological, nutrient distribution, and targeted metabolomics analyses. SA had no impact on plant physiological parameters, however, 30+ dysregulated metabolites and 15+ perturbed metabolomic pathways were identified in wheat leaves and roots. Cu(OH)2 NMs resulted in an accumulation of 649.8 μg/g Cu in leaves; even with minimal Cu translocation, levels of 27 metabolites were significantly changed in roots. Due to the low dissolution of Cu(OH)2 NMs in SA, the low concentration of Cu IONs induced minimal plant response. In contrast, given the substantial dissolution of MoO3 NMs (35.8%), the corresponding high levels of Mo IONs resulted in significant metabolite reprogramming (30+ metabolites dysregulated). Aspartic acid, proline, chlorogenic acid, adenosine, ascorbic acid, phenylalanine, and lysine were significantly upregulated for MoO3 NMs, yet downregulated under Mo IONs condition. Surprisingly, Cu(OH)2 NMs stimulated wheat plant tissues more than MoO3 NMs. The glyoxylate/dicarboxylate metabolism (in leaves) and valine/leucine/isoleucine biosynthesis (in roots) uniquely responded to Cu(OH)2 NMs. Findings from this study provide novel insights on the use of surfactants to enhance the foliar application of nanoagrochemicals.
Keywords: metabolomics; nanoagrochemicals; nanomaterials; surfactants; wheat.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
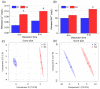

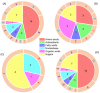

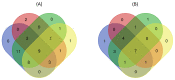
 wheat leaves exposed to Cu(OH)2 NMs;
wheat leaves exposed to Cu(OH)2 NMs;  wheat leaves exposed to MoO3 NMs;
wheat leaves exposed to MoO3 NMs;  wheat roots exposed to Cu(OH)2 NMs;
wheat roots exposed to Cu(OH)2 NMs;  wheat roots exposed to MoO3 NMs.
wheat roots exposed to MoO3 NMs.References
-
- Alshaal T., El-Ramady H. Foliar application: From plant nutrition to biofortification. Environ. Biodivers. Soil Secur. 2017;1:71–83. doi: 10.21608/jenvbs.2017.1089.1006. - DOI
-
- Tan W., Gao Q., Deng C., Wang Y., Lee W.-Y., Hernandez-Viezcas J.A., Peralta-Videa J.R., Gardea-Torresdey J.L. Foliar exposure of Cu(OH)2 nanopesticide to basil (Ocimum basilicum): Variety-dependent copper translocation and biochemical responses. J. Agric. Food Chem. 2018;66:3358–3366. doi: 10.1021/acs.jafc.8b00339. - DOI - PubMed
-
- Adisa I.O., Pullagurala V.L.R., Peralta-Videa J.R., Dimkpa C.O., Elmer W.H., Gardea-Torresdey J.L., White J.C. Recent advances in nano-enabled fertilizers and pesticides: A critical review of mechanisms of action. Environ. Sci. Nano. 2019;6:2002–2030. doi: 10.1039/C9EN00265K. - DOI

