CdIn2S4/In(OH)3/NiCr-LDH Multi-Interface Heterostructure Photocatalyst for Enhanced Photocatalytic H2 Evolution and Cr(VI) Reduction
- PMID: 34835886
- PMCID: PMC8619374
- DOI: 10.3390/nano11113122
CdIn2S4/In(OH)3/NiCr-LDH Multi-Interface Heterostructure Photocatalyst for Enhanced Photocatalytic H2 Evolution and Cr(VI) Reduction
Abstract
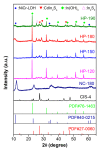
The development of highly active and stable photocatalysts, an effective way to remediate environment pollution and alleviate energy shortages, remains a challenging issue. In this work, a CdIn2S4/In(OH)3 nanocomposite was deposited in-situ on NiCr-LDH nanosheets by a simple hydrothermal method, and the obtained CdIn2S4/In(OH)3/NiCr-LDH heterostructure photocatalysts with multiple intimate-contact interfaces exhibited better photocatalytic activity. The photocatalytic H2 evolution rate of CdIn2S4/In(OH)3/NiCr-LDH increased to 10.9 and 58.7 times that of the counterparts CdIn2S4 and NiCr-LDH, respectively. Moreover, the photocatalytic removal efficiency of Cr(VI) increased from 6% for NiCr-LDH and 75% for CdIn2S4 to 97% for CdIn2S4/In(OH)3/NiCr-LDH. The enhanced photocatalytic performance was attributed to the formation of multi-interfaces with strong interfacial interactions and staggered band alignments, which offered multiple pathways for carrier migration, thus promoting the separation efficiency of photo-excited electrons and holes. This study demonstrates a facile method to fabricate inexpensive and efficient heterostructure photocatalysts for solving environmental problems.
Keywords: CdIn2S4; In(OH)3; LDHs; charge separation; nano-heterostructures; photocatalysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Dawood F., Anda M., Shafiullah G.M. Hydrogen production for energy: An overview. Int. J. Hydrog. Energ. 2020;45:3847–3869. doi: 10.1016/j.ijhydene.2019.12.059. - DOI
-
- Wang Y., Diaz D.F.R., Chen K.S., Wang Z., Adroher X.C. Materials, technological status, and fundamentals of PEM fuel cells—A review. Mater. Today. 2020;32:178–203. doi: 10.1016/j.mattod.2019.06.005. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

