Time-Limited Eating and Continuous Glucose Monitoring in Adolescents with Obesity: A Pilot Study
- PMID: 34835953
- PMCID: PMC8624400
- DOI: 10.3390/nu13113697
Time-Limited Eating and Continuous Glucose Monitoring in Adolescents with Obesity: A Pilot Study
Abstract
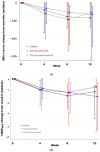
Due to its simplicity, time-limited eating (TLE) may represent a more feasible approach for treating adolescents with obesity compared to other caloric restriction regimens. This pilot study examines the feasibility and safety of TLE combined with continuous glucose monitoring (CGM) in adolescents. Fifty adolescents with BMI ≥95th percentile were recruited to complete a 12-week study. All received standard nutritional counseling, wore a CGM daily, and were randomized to: (1) Prolonged eating window: 12 h eating/12 h fasting + blinded CGM; (2) TLE (8 h eating/16 h fasting, 5 days per week) + blinded CGM; (3) TLE + real-time CGM feedback. Recruitment, retention, and adherence were recorded as indicators of feasibility. Weight loss, dietary intake, physical activity, eating behaviors, and quality of life over the course of the intervention were explored as secondary outcomes. Forty-five participants completed the study (16.4 ± 1.3 years, 64% female, 49% Hispanic, 75% public insurance). There was high adherence to prescribed eating windows (TLE 5.2 d/wk [SD 1.1]; control 6.1 d/wk [SD 1.4]) and daily CGM wear (5.85 d/wk [SD 4.8]). Most of the adolescents (90%) assigned to TLE reported that limiting their eating window and wearing a CGM was feasible without negative impact on daily functioning or adverse events. There were no between-group difference in terms of weight loss, energy intake, quality of life, physical activity, or eating behaviors. TLE combined with CGM appears feasible and safe among adolescents with obesity. Further investigation in larger samples, with a longer intervention duration and follow-up assessments are needed.
Keywords: adolescents; continuous glucose monitor; intermittent fasting; obesity; pediatrics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Copeland K.C., Zeitler P., Geffner M., Guandalini C., Higgins J., Hirst K., Kaufman F.R., Linder B., Marcovina S., McGuigan P., et al. Characteristics of adolescents and youth with recent-onset type 2 diabetes: The TODAY cohort at baseline. J. Clin. Endocrinol. Metab. 2011;96:159–167. doi: 10.1210/jc.2010-1642. - DOI - PMC - PubMed
-
- Marcus M.D., Wilfley D.E., El Ghormli L., Zeitler P., Linder B., Hirst K., Ievers-Landis C.E., van Buren D.J., Walders-Abramson N., TODAY Study Group Weight change in the management of youth-onset type 2 diabetes: The TODAY clinical trial experience. Pediatr. Obes. 2017;12:337–345. doi: 10.1111/ijpo.12148. - DOI - PMC - PubMed
-
- August G.P., Caprio S., Fennoy I., Freemark M., Kaufman F.R., Lustig R.H., Silverstein J.H., Speiser P.W., Styne D.M., Montori V.M. Prevention and treatment of pediatric obesity: An Endocrine Society clinical practice guideline based on expert opinion. J. Clin. Endocrinol. Metab. 2008;93:4576–4599. doi: 10.1210/jc.2007-2458. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
- UL1TR001855/National Center for Advancing Translational Science (NCATS) of the U.S. National Institutes of Health
- R01HD092483/the Eunice Kennedy Shriver National Institute of Child Health and Human Development
- UL1 TR001855/TR/NCATS NIH HHS/United States
- R01 CA258222/CA/NCI NIH HHS/United States
- U54MD000502/National Institute on Minority Health and Health Disparities (NIMHD) Obesity Health Disparities Research Center
LinkOut - more resources
Full Text Sources

