Vitamins as Possible Cancer Biomarkers: Significance and Limitations
- PMID: 34836171
- PMCID: PMC8622959
- DOI: 10.3390/nu13113914
Vitamins as Possible Cancer Biomarkers: Significance and Limitations
Abstract
The Western-style diet, which is common in developed countries and spreading into developing countries, is unbalanced in many respects. For instance, micronutrients (vitamins A, B complex, C, D, E, and K plus iron, zinc, selenium, and iodine) are generally depleted in Western food (causing what is known as 'hidden hunger'), whereas some others (such as phosphorus) are added beyond the daily allowance. This imbalance in micronutrients can induce cellular damage that can increase the risk of cancer. Interestingly, there is a large body of evidence suggesting a strong correlation between vitamin intake as well as vitamin blood concentrations with the occurrence of certain types of cancer. The direction of association between the concentration of a given vitamin and cancer risk is tumor specific. The present review summarized the literature regarding vitamins and cancer risk to assess whether these could be used as diagnostic or prognostic markers, thus confirming their potential as biomarkers. Despite many studies that highlight the importance of monitoring vitamin blood or tissue concentrations in cancer patients and demonstrate the link between vitamin intake and cancer risk, there is still an urgent need for more data to assess the effectiveness of vitamins as biomarkers in the context of cancer. Therefore, this review aims to provide a solid basis to support further studies on this promising topic.
Keywords: cancer biomarker; cancer risk; vitamin A; vitamin B complex; vitamin C; vitamin D; vitamin E; vitamin K.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
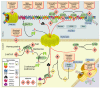

References
-
- Vallgårda S., Nielsen M.E.J., Hansen A.K.K., Cathaoir K.Ó., Hartlev M., Holm L., Christensen B.J., Jensen J.D., Sørensen T.I.A., Sandøe P. Should Europe follow the US and declare obesity a disease?: A discussion of the so-called utilitarian argument. Eur. J. Clin. Nutr. 2017;71:1263–1267. doi: 10.1038/ejcn.2017.103. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

