Comparative efficacy and safety of first-line treatments for advanced non-small cell lung cancer with ALK-rearranged: a meta-analysis of clinical trials
- PMID: 34836510
- PMCID: PMC8620528
- DOI: 10.1186/s12885-021-08977-0
Comparative efficacy and safety of first-line treatments for advanced non-small cell lung cancer with ALK-rearranged: a meta-analysis of clinical trials
Abstract
Background: Whereas there are many pharmacological interventions prescribed for patients with advanced anaplastic lymphoma kinase (ALK)- rearranged non-small cell lung cancer (NSCLC), comparative data between novel generation ALK-tyrosine kinase inhibitors (TKIs) remain scant. Here, we indirectly compared the efficacy and safety of first-line systemic therapeutic options used for the treatment of ALK-rearranged NSCLC.
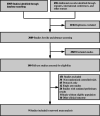
Methods: We included all phase 2 and 3 randomised controlled trials (RCTs) comparing any two or three treatment options. Eligible studies reported at least one of the following outcomes: progression free survival (PFS), overall survival (OS), objective response rate (ORR), or adverse events of grade 3 or higher (Grade ≥ 3 AEs). Subgroup analysis was conducted according to central nervous system (CNS) metastases.
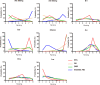
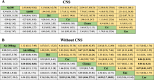
Results: A total of 9 RCTs consisting of 2484 patients with 8 treatment options were included in the systematic review. Our analysis showed that alectinib (300 mg and 600 mg), brigatinib, lorlatinib and ensartinib yielded the most favorable PFS. Whereas there was no significant OS or ORR difference among the ALK-TKIs. According to Bayesian ranking profiles, lorlatinib, alectinib 600 mg and alectinib 300 mg had the best PFS (63.7%), OS (35.9%) and ORR (37%), respectively. On the other hand, ceritinib showed the highest rate of severe adverse events (60%).
Conclusion: Our analysis indicated that alectinib and lorlatinib might be associated with the best therapeutic efficacy in first-line treatment for major population of advanced NSCLC patients with ALK-rearrangement. However, since there is little comparative evidence on the treatment options, there is need for relative trials to fully determine the best treatment options as well as the rapidly evolving treatment landscape.
Keywords: ALK; First-line treatment; Lung cancer; Network meta-analysis.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Sung H, Ferlay J, Siegel R, Laversanne M, Soerjomataram I, Jemal A, Bray FJCacjfc: Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. 2021, 71(3):209–249. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

