Extracellular haem utilization by the opportunistic pathogen Pseudomonas aeruginosa and its role in virulence and pathogenesis
- PMID: 34836613
- PMCID: PMC8928441
- DOI: 10.1016/bs.ampbs.2021.07.004
Extracellular haem utilization by the opportunistic pathogen Pseudomonas aeruginosa and its role in virulence and pathogenesis
Abstract
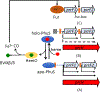
Iron is an essential micronutrient for all bacteria but presents a significant challenge given its limited bioavailability. Furthermore, iron's toxicity combined with the need to maintain iron levels within a narrow physiological range requires integrated systems to sense, regulate and transport a variety of iron complexes. Most bacteria encode systems to chelate and transport ferric iron (Fe3+) via siderophore receptor mediated uptake or via cytoplasmic energy dependent transport systems. Pathogenic bacteria have further lowered the barrier to iron acquisition by employing systems to utilize haem as a source of iron. Haem, a lipophilic and toxic molecule, presents a significant challenge for transport into the cell. As such pathogenic bacteria have evolved sophisticated cell surface signaling (CSS) and transport systems to sense and obtain haem from the host. Once internalized haem is cleaved by both oxidative and non-oxidative mechanisms to release iron. Herein we summarize our current understanding of the mechanism of haem sensing, uptake and utilization in Pseudomonas aeruginosa, its role in pathogenesis and virulence, and the potential of these systems as antimicrobial targets.
Keywords: Bacterial pathogenesis; Biliverdin; ECF σ-factor systems; Haem uptake and utilization; Iron and haem regulated sRNAs; Iron homeostasis; Pseudomonas aeruginosa; Transcriptional regulation.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Disclosure statement The authors are not aware of any affiliations, funding or financial interests that might be perceived as influencing the objectivity of this review.
Figures

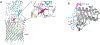

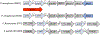




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

