A Perspective on the Potential Involvement of Impaired Proteostasis in Neuropsychiatric Disorders
- PMID: 34836635
- PMCID: PMC8792182
- DOI: 10.1016/j.biopsych.2021.09.001
A Perspective on the Potential Involvement of Impaired Proteostasis in Neuropsychiatric Disorders
Abstract
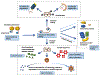
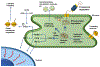
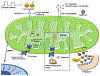
Recent genetic approaches have demonstrated that genetic factors contribute to the pathologic origins of neuropsychiatric disorders. Nevertheless, the exact pathophysiological mechanism for most cases remains unclear. Recent studies have demonstrated alterations in pathways of protein homeostasis (proteostasis) and identified several proteins that are misfolded and/or aggregated in the brains of patients with neuropsychiatric disorders, thus providing early evidence that disrupted proteostasis may be a contributing factor to their pathophysiology. Unlike neurodegenerative disorders in which massive neuronal and synaptic losses are observed, proteostasis impairments in neuropsychiatric disorders do not lead to robust neuronal death, but rather likely act via loss- and gain-of-function effects to disrupt neuronal and synaptic functions. Furthermore, abnormal activation of or overwhelmed endoplasmic reticulum and mitochondrial quality control pathways may exacerbate the pathophysiological changes initiated by impaired proteostasis, as these organelles are critical for proper neuronal functions and involved in the maintenance of proteostasis. This perspective article reviews recent findings implicating proteostasis impairments in the pathophysiology of neuropsychiatric disorders and explores how neuronal and synaptic functions may be impacted by disruptions in protein homeostasis. A greater understanding of the contributions by proteostasis impairment in neuropsychiatric disorders will help guide future studies to identify additional candidate proteins and new targets for therapeutic development.
Keywords: ER stress; mitochondria; neuropsychiatric disorders; protein aggregation; protein misfolding; proteostasis.
Copyright © 2021 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Financial Disclosures
The authors reported no biomedical financial interests or potential conflicts of interest.
Figures
References
-
- Desai PR, Lawson KA, Barner JC, Rascati KL (2013): Estimating the direct and indirect costs for community- dwelling patients with schizophrenia. J Pharm Heal Serv Res 4: 187–194.
-
- WU EQ, SHI L, BIRNBAUM H, HUDSON T, KESSLER R (2006): Annual prevalence of diagnosed schizophrenia in the USA: a claims data analysis approach. Psychol Med 36: 1535–1540. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

