SARS-CoV-2 infection and oxidative stress: Pathophysiological insight into thrombosis and therapeutic opportunities
- PMID: 34836751
- PMCID: PMC8591899
- DOI: 10.1016/j.cytogfr.2021.11.001
SARS-CoV-2 infection and oxidative stress: Pathophysiological insight into thrombosis and therapeutic opportunities
Abstract
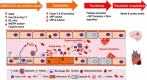
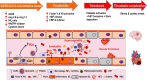
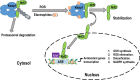
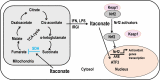
The current coronavirus disease 2019 (COVID-19) pandemic has presented unprecedented challenges to global health. Although the majority of COVID-19 patients exhibit mild-to-no symptoms, many patients develop severe disease and need immediate hospitalization, with most severe infections associated with a dysregulated immune response attributed to a cytokine storm. Epidemiological studies suggest that overall COVID-19 severity and morbidity correlate with underlying comorbidities, including diabetes, obesity, cardiovascular diseases, and immunosuppressive conditions. Patients with such comorbidities exhibit elevated levels of reactive oxygen species (ROS) and oxidative stress caused by an increased accumulation of angiotensin II and by activation of the NADPH oxidase pathway. Moreover, accumulating evidence suggests that oxidative stress coupled with the cytokine storm contribute to COVID-19 pathogenesis and immunopathogenesis by causing endotheliitis and endothelial cell dysfunction and by activating the blood clotting cascade that results in blood coagulation and microvascular thrombosis. In this review, we survey the mechanisms of how severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) induces oxidative stress and the consequences of this stress on patient health. We further shed light on aspects of the host immunity that are crucial to prevent the disease during the early phase of infection. A better understanding of the disease pathophysiology as well as preventive measures aimed at lowering ROS levels may pave the way to mitigate SARS-CoV-2-induced complications and decrease mortality.
Keywords: COVID-19; Oxidative stress; Pathophysiology; SARS-CoV-2; Thrombosis.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Figures




References
-
- Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. - PubMed
-
- Zhou Y., Zhang Z., Tian J., Xiong S. Risk factors associated with disease progression in a cohort of patients infected with the 2019 novel coronavirus. Ann. Palliat. Med. 2020;9(2):428–436. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

