Follicular dendritic cell sarcoma
- PMID: 34837090
- PMCID: PMC8720404
- DOI: 10.32074/1591-951X-331
Follicular dendritic cell sarcoma
Abstract
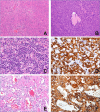
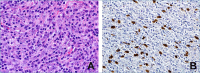
Follicular dendritic cells (FDC) are mesenchymal-derived dendritic cells located in B-follicles where they play a pivotal role in triggering and maintaining B-cell adaptive immune response. In 1986 Dr. Juan Rosai first reported a series of neoplasms showing features of FDC and defined it as Follicular Dendritic Cell Tumor, subsequently renamed as "sarcoma" (FDCS). In its seminal and subsequent articles Rosai and colleagues highlighted the heterogeneous microscopic appearance of FDCS and its immunohistochemical and ultrastructural features.
FDCS mostly occurs in extranodal sites (79.4% of cases) and lymph nodes (15.1%); in about 7%-10% of cases it is associated with hyaline-vascular Castleman disease. Given its significant growth pattern and cytological variability, FDCS can be confused with various neoplasms and even inflammatory processes. The diagnosis requires the use of a broad spectrum of FDC markers (e.g. CD21, CD23, CD35, clusterin, CXCL13, podoplanin), particularly considering that tumor antigen-loss is frequent. The inflammatory-pseudotumor-like (IPT-like) variant of FDCS, in addition to its peculiar histopathological and clinical features, is characterized by positivity of tumor cells for Epstein-Barr virus, representing a diagnostic requisite.
No distinctive genetic and molecular anomalies have been identified in FDCS. It often carries an aberrant clonal karyotype and chromosomal structural alterations, frequently involving onco-suppressor genes. Direct or next generation sequencing showed alterations on genes belonging to the NF-κB regulatory pathway and cell-cycle regulators. In contrast to hematopoietic-derived histiocytic and dendritic cells tumors, FDCS typically lacks mutations in genes related to the MAPK pathway.
FDCS recurs locally in 28% and metastasizes in 27% of cases. Extent of the disease, surgical resectability and histopathological features are significantly associated with the outcome. IPT-like FDCS behaves as an indolent tumor, even if it often recurs locally over years.
Complete surgical excision is the gold standard of treatment. Data on targeted therapies (e.g.: tyrosine kinase inhibitors) or immune checkpoint inhibitors are very limited and responses are variable. A better understanding of the molecular drivers of this tumor may lead to potential new therapeutic strategies.
Keywords: diagnosis; follicular dendritic cell sarcoma; mutations; personalized medicine.
Copyright © 2021 Società Italiana di Anatomia Patologica e Citopatologia Diagnostica, Divisione Italiana della International Academy of Pathology.
Conflict of interest statement
The Authors declare no conflict of interest.
Figures




References
-
- Perez-Ordonez B, Erlandson RA, Rosai J. Follicular dendritic cell tumor: report of 13 additional cases of a distinctive entity. Am J Surg Pathol 1996;20:944-955. https://doi.org/10.1097/00000478-199608000-00003 10.1097/00000478-199608000-00003 - DOI - PubMed
-
- Perez-Ordonez B, Rosai J. Follicular dendritic cell tumor: review of the entity. Semin Diagn Pathol 1998;15:144-154. - PubMed
-
- Aguzzi A, Kranich J, Krautler NJ. Follicular dendritic cells: origin, phenotype, and function in health and disease. Trends Immunol 2014;35:105-113. https://doi.org/10.1016/j.it.2013.11.001 10.1016/j.it.2013.11.001 - DOI - PubMed
-
- Facchetti F, Lorenzi L. Follicular dendritic cells and related sarcoma. Semin Diagn Pathol 2016;33:159-166. https://doi.org/10.1053/j.semdp.2016.05.002 10.1053/j.semdp.2016.05.002 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

