A Microclip Peripheral Nerve Interface (μcPNI) for Bioelectronic Interfacing with Small Nerves
- PMID: 34837353
- PMCID: PMC8787429
- DOI: 10.1002/advs.202102945
A Microclip Peripheral Nerve Interface (μcPNI) for Bioelectronic Interfacing with Small Nerves
Abstract
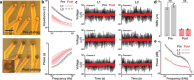
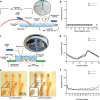
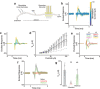
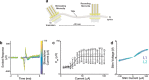
Peripheral nerves carry sensory (afferent) and motor (efferent) signals between the central nervous system and other parts of the body. The peripheral nervous system (PNS) is therefore rich in targets for therapeutic neuromodulation, bioelectronic medicine, and neuroprosthetics. Peripheral nerve interfaces (PNIs) generally suffer from a tradeoff between selectivity and invasiveness. This work describes the fabrication, evaluation, and chronic implantation in zebra finches of a novel PNI that breaks this tradeoff by interfacing with small nerves. This PNI integrates a soft, stretchable microelectrode array with a 2-photon 3D printed microclip (μcPNI). The advantages of this μcPNI compared to other designs are: a) increased spatial resolution due to bi-layer wiring of the electrode leads, b) reduced mismatch in biomechanical properties with the nerve, c) reduced disturbance to the host tissue due to the small size, d) elimination of sutures or adhesives, e) high circumferential contact with small nerves, f) functionality under considerable strain, and g) graded neuromodulation in a low-threshold stimulation regime. Results demonstrate that the μcPNIs are electromechanically robust, and are capable of reliably recording and stimulating neural activity in vivo in small nerves. The μcPNI may also inform the development of new optical, thermal, ultrasonic, or chemical PNIs as well.
Keywords: 3D printing; bioelectronic medicine; peripheral nerve interfaces; stretchable microelectrode arrays.
© 2021 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Bouton C., J. Intern. Med. 2017, 282, 37. - PubMed
-
- Raspopovic S., Capogrosso M., Petrini F. M., Bonizzato M., Rigosa J., Di Pino G., Carpaneto J., Controzzi M., Boretius T., Fernandez E., Granata G., Oddo C. M., Citi L., Ciancio A. L., Cipriani C., Carrozza M. C., Jensen W., Guglielmelli E., Stieglitz T., Rossini P. M., Micera S., Sci. Transl. Med. 2014, 6, 222ra19. - PubMed
-
- Birmingham K., Gradinaru V., Anikeeva P., Grill W. M., Pikov V., McLaughlin B., Pasricha P., Weber D., Ludwig K., Famm K., Nat. Rev. Drug Discovery 2014, 13, 399. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
