Comparing efficacy and safety of different doses of dexamethasone in the treatment of COVID-19: a three-arm randomized clinical trial
- PMID: 34837648
- PMCID: PMC8627167
- DOI: 10.1007/s43440-021-00341-0
Comparing efficacy and safety of different doses of dexamethasone in the treatment of COVID-19: a three-arm randomized clinical trial
Abstract
Background and objectives: Corticosteroids are commonly used in the treatment of hospitalized patients with COVID-19. The goals of the present study were to compare the efficacy and safety of different doses of dexamethasone in the treatment of patients with a diagnosis of moderate to severe COVID-19.
Methods: Hospitalized patients with a diagnosis of moderate to severe COVID-19 were assigned to intravenous low-dose (8 mg once daily), intermediate-dose (8 mg twice daily) or high-dose (8 mg thrice daily) dexamethasone for up to 10 days or until hospital discharge. Clinical response, 60-day survival and adverse effects were the main outcomes of the study.
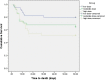
Results: In the competing risk survival analysis, patients in the low-dose group had a higher clinical response than the high-dose group when considering death as a competing risk (HR = 2.03, 95% CI: 1.23-3.33, p = 0.03). Also, the survival was significantly longer in the low-dose group than the high-dose group (HR = 0.36, 95% CI = 0.15-0.83, p = 0.02). Leukocytosis and hyperglycemia were the most common side effects of dexamethasone. Although the incidence was not significantly different between the groups, some adverse effects were numerically higher in the intermediate-dose and high-dose groups than in the low-dose group.
Conclusions: Higher doses of dexamethasone not only failed to improve efficacy but also resulted in an increase in the number of adverse events and worsen survival in hospitalized patients with moderate to severe COVID-19 compared to the low-dose dexamethasone. (IRCT20100228003449N31).
Keywords: COVID-19; Dexamethasone; High-dose; Intermediate-dose; Low-dose.
© 2021. The Author(s) under exclusive licence to Maj Institute of Pharmacology Polish Academy of Sciences.
Conflict of interest statement
There is no conflict of interest for authors to declare.
Figures

References
-
- World Health Organization, COVID-19 Weekly Epidemiological Update, 11 October 2021. 2021. https://www.who.int/publications/m/item/weekly-epidemiological-update-on.... Accessed 14 Oct 2021.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
