Sequential first-line treatment with nab-paclitaxel/gemcitabine and FOLFIRINOX in metastatic pancreatic adenocarcinoma: GABRINOX phase Ib-II controlled clinical trial
- PMID: 34837745
- PMCID: PMC8637474
- DOI: 10.1016/j.esmoop.2021.100318
Sequential first-line treatment with nab-paclitaxel/gemcitabine and FOLFIRINOX in metastatic pancreatic adenocarcinoma: GABRINOX phase Ib-II controlled clinical trial
Abstract
Background: Nab-paclitaxel/gemcitabine (AG) and FOLFIRINOX (FFX) are promising drugs in metastatic pancreatic cancer (MPC). This study evaluated a new first-line sequential treatment (AG followed by FFX) in MPC that might overcome resistance to primary therapy and delay tumor progression.
Patients and methods: Patients with histologically/cytologically confirmed MPC were included in a multicentric trial receiving AG (day 1, 8 and 15) followed by FFX (day 29 and 43). In phase Ib, three dose-levels were tested for maximum tolerated dose (MTD) and recommended phase II dose. In phase II, the main outcome was the objective response rate (ORR) and secondarily safety, progression-free survival (PFS) and overall survival (OS).
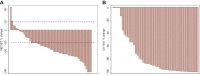
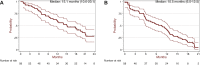
Results: In phase Ib, we included 33 patients (31 assessable) of median age 61.0 years (range 42-75 years) and represented by 54.8% males. Five dose-limiting toxicities were reported without any death. The main grade 3/4 toxicities were neutropenia with spontaneous resolution (35.5%/32.3%), venous thromboembolism (grade 3: 22.6%) and thrombopenia (grade 3: 29.0%), while the MTD was not reached. In phase II, we included 58 patients of median age 60 years (range 34-72 years), 50% males and with Eastern Cooperative Oncology Group stage score 0 and 1 of 37.9% and 62.1%, respectively. They received a median of 4 (1-9) cycles in 8.5 months (0.5-19.8 months). The ORR was 64.9% [95% confidence interval (CI) 51.1% to 77.1%], and neurotoxicity was remarkably low. The main grade 3-4 toxicities were venous thromboembolism, thrombopenia, neutropenia/febrile neutropenia, nausea, diarrhea, weight loss and asthenia without any death. Tumor response was complete in 3.5% and partial in 61.4%, while disease was stable in 19.3% and progressive in 15.8% of patients. The median PFS was 10.5 months (95% CI 6.0-12.5 months) and median OS was 15.1 months (95% CI 10.6-20.1 months).
Conclusion: Sequential AG and FFX showed acceptable toxicity as first-line treatment with no limiting neurotoxicity, while high response rate and survival justify randomized trials.
Keywords: FOLFIRINOX; adenocarcinoma; gemcitabine; metastasis; nab-paclitaxel; pancreatic cancer.
Copyright © 2021 Institut régional du Cancer de Montepllier (ICM). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Disclosure The authors have declared no conflicts of interest.
Figures

References
-
- Rahib L., Smith B.D., Aizenberg R., et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. - PubMed
-
- Ferlay J., Partensky C., Bray F. More deaths from pancreatic cancer than breast cancer in the EU by 2017. Acta Oncol Stockh Swed. 2016;55:1158–1160. - PubMed
-
- Ducreux M., Cuhna A.S., Caramella C., et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(suppl 5):v56–v68. - PubMed
-
- Bray F., Ferlay J., Soerjomataram I., et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

