The disulfide bond Cys2724-Cys2774 in the C-terminal cystine knot domain of von Willebrand factor is critical for its dimerization and secretion
- PMID: 34838051
- PMCID: PMC8626975
- DOI: 10.1186/s12959-021-00348-w
The disulfide bond Cys2724-Cys2774 in the C-terminal cystine knot domain of von Willebrand factor is critical for its dimerization and secretion
Abstract
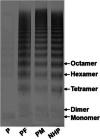
Background: Type 3 von Willebrand disease (VWD) exhibits severe hemorrhagic tendency with complicated pathogenesis. The C-terminal cystine knot (CTCK) domain plays an important role in the dimerization and secretion of von Willebrand factor (VWF). The CTCK domain has four intrachain disulfide bonds including Cys2724-Cys2774, Cys2739-Cys2788, Cys2750-Cys2804 and Cys2754-Cys2806, and the single cysteine mutation in Cys2739-Cys2788, Cys2750-Cys2804 and Cys2754-Cys2806 result in type 3 VWD, demonstrating the crucial role of these three disulfide bonds in VWF biosynthesis, however, the role of the remaining disulfide bond Cys2724-Cys2774 remains unclear.
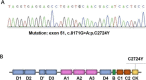
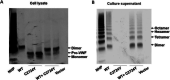
Method and results: In this study, by the next-generation sequencing we found a missense mutation a c.8171G>A (C2724Y) in the CTCK domain of VWF allele in a patient family with type 3 VWD. In vitro, VWF C2724Y protein was expressed normally in HEK-293T cells but did not form a dimer or secrete into cell culture medium, suggesting that C2724 is critical for the VWF dimerization, and thus for VWF multimerization and secretion.
Conclusions: Our findings provide the first genetic evidence for the important role of Cys2724-Cys2774 in VWF biosynthesis and secretion. Therefore, all of the four intrachain disulfide bonds in CTCK monomer contribute to VWF dimerization and secretion.
Keywords: cystine knot domain; disulfide bond; multimerization; von Willebrand disease (VWD); von Willebrand factor (VWF).
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
The authors declare no conflicts of interest.
Figures

Similar articles
-
Differential effects of the loss of intrachain- versus interchain-disulfide bonds in the cystine-knot domain of von Willebrand factor on the clinical phenotype of von Willebrand disease.Thromb Haemost. 2006 Dec;96(6):717-24. doi: 10.1160/th06-08-0460. Thromb Haemost. 2006. PMID: 17139364
-
VWF-Gly2752Ser, a novel non-cysteine substitution variant in the CK domain, exhibits severe secretory impairment by hampering C-terminal dimer formation.J Thromb Haemost. 2022 Aug;20(8):1784-1796. doi: 10.1111/jth.15746. Epub 2022 May 22. J Thromb Haemost. 2022. PMID: 35491445
-
Expression and characterization of von Willebrand factor dimerization defects in different types of von Willebrand disease.Blood. 2001 Apr 1;97(7):2059-66. doi: 10.1182/blood.v97.7.2059. Blood. 2001. PMID: 11264172
-
Laboratory diagnosis of von Willebrand disease type 1/2E (2A subtype IIE), type 1 Vicenza and mild type 1 caused by mutations in the D3, D4, B1-B3 and C1-C2 domains of the von Willebrand factor gene. Role of von Willebrand factor multimers and the von Willebrand factor propeptide/antigen ratio.Acta Haematol. 2009;121(2-3):128-38. doi: 10.1159/000214853. Epub 2009 Jun 8. Acta Haematol. 2009. PMID: 19506359 Review.
-
Diagnostic Differentiation of von Willebrand Disease Types 1 and 2 by von Willebrand Factor Multimer Analysis and DDAVP Challenge Test.Clin Appl Thromb Hemost. 2017 Sep;23(6):518-531. doi: 10.1177/1076029616647157. Epub 2016 Jul 21. Clin Appl Thromb Hemost. 2017. PMID: 27443694 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

