Hepatic small extracellular vesicles promote microvascular endothelial hyperpermeability during NAFLD via novel-miRNA-7
- PMID: 34838052
- PMCID: PMC8626954
- DOI: 10.1186/s12951-021-01137-3
Hepatic small extracellular vesicles promote microvascular endothelial hyperpermeability during NAFLD via novel-miRNA-7
Abstract
Background: A recent study has reported that patients with nonalcoholic fatty liver disease (NAFLD) are more susceptible to coronary microvascular dysfunction (CMD), which may predict major adverse cardiac events. However, little is known regarding the causes of CMD during NAFLD. In this study, we aimed to explore the role of hepatic small extracellular vesicles (sEVs) in regulating the endothelial dysfunction of coronary microvessels during NAFLD.
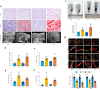
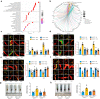
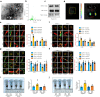
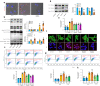
Results: We established two murine NAFLD models by feeding mice a methionine-choline-deficient (MCD) diet for 4 weeks or a high-fat diet (HFD) for 16 weeks. We found that the NOD-like receptor family, pyrin domain containing 3 (NLRP3) inflammasome-dependent endothelial hyperpermeability occurred in coronary microvessels during both MCD diet and HFD-induced NAFLD. The in vivo and in vitro experiments proved that novel-microRNA(miR)-7-abundant hepatic sEVs were responsible for NLRP3 inflammasome-dependent endothelial barrier dysfunction. Mechanistically, novel-miR-7 directly targeted lysosomal associated membrane protein 1 (LAMP1) and promotes lysosomal membrane permeability (LMP), which in turn induced Cathepsin B-dependent NLRP3 inflammasome activation and microvascular endothelial hyperpermeability. Conversely, a specific novel-miR-7 inhibitor markedly improved endothelial barrier integrity. Finally, we proved that steatotic hepatocyte was a significant source of novel-miR-7-contained hepatic sEVs, and steatotic hepatocyte-derived sEVs were able to promote NLRP3 inflammasome-dependent microvascular endothelial hyperpermeability through novel-miR-7.
Conclusions: Hepatic sEVs contribute to endothelial hyperpermeability in coronary microvessels by delivering novel-miR-7 and targeting the LAMP1/Cathepsin B/NLRP3 inflammasome axis during NAFLD. Our study brings new insights into the liver-to-microvessel cross-talk and may provide a new diagnostic biomarker and treatment target for microvascular complications of NAFLD.
Keywords: Inter-organ communication; Liver inflammation; Microvascular endothelial dysfunction; NLRP3 inflammasome; Tight junction.
© 2021. The Author(s).
Conflict of interest statement
All authors declared no conflict of interests.
Figures


References
MeSH terms
Substances
Grants and funding
- 2017YFC1700400/the National Key Research and Development Program of China
- 2017YFC1700404/the National Key Research and Development Program of China
- 20181B030306027/the Guangdong Natural Science Funds for Distinguished Young Scholars
- 2018A030310622/the Basic Research Project of Guangdong Province
- 202103000044/the Major Program of Science and Technology Program of Guangzhou
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

