The Scleroderma Patient-centered Intervention Network Self-Management (SPIN-SELF) Program: protocol for a two-arm parallel partially nested randomized controlled feasibility trial with progression to full-scale trial
- PMID: 34838105
- PMCID: PMC8626736
- DOI: 10.1186/s13063-021-05827-z
The Scleroderma Patient-centered Intervention Network Self-Management (SPIN-SELF) Program: protocol for a two-arm parallel partially nested randomized controlled feasibility trial with progression to full-scale trial
Abstract
Background: Systemic sclerosis (scleroderma; SSc) is a rare autoimmune connective tissue disease. We completed an initial feasibility trial of an online self-administered version of the Scleroderma Patient-centered Intervention Network Self-Management (SPIN-SELF) Program using the cohort multiple randomized controlled trial (RCT) design. Due to low intervention offer uptake, we will conduct a new feasibility trial with progression to full-scale trial, using a two-arm parallel, partially nested RCT design. The SPIN-SELF Program has also been revised to include facilitator-led videoconference group sessions in addition to online material. We will test the group-based intervention delivery format, then evaluate the effect of the SPIN-SELF Program on disease management self-efficacy (primary) and patient activation, social appearance anxiety, and functional health outcomes (secondary).
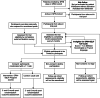
Methods: This study is a feasibility trial with progression to full-scale RCT, pending meeting pre-defined criteria, of the SPIN-SELF Program. Participants will be recruited from the ongoing SPIN Cohort ( http://www.spinsclero.com/en/cohort ) and via social media and partner patient organizations. Eligible participants must have SSc and low to moderate disease management self-efficacy (Self-Efficacy for Managing Chronic Disease (SEMCD) Scale score ≤ 7.0). Participants will be randomized (1:1 allocation) to the group-based SPIN-SELF Program or usual care for 3 months. The primary outcome in the full-scale trial will be disease management self-efficacy based on SEMCD Scale scores at 3 months post-randomization. Secondary outcomes include SEMCD scores 6 months post-randomization plus patient activation, social appearance anxiety, and functional health outcomes at 3 and 6 months post-randomization. We will include 40 participants to assess feasibility. At the end of the feasibility portion, stoppage criteria will be used to determine if the trial procedures or SPIN-SELF Program need important modifications, thereby requiring a re-set for the full-scale trial. Otherwise, the full-scale RCT will proceed, and outcome data from the feasibility portion will be utilized in the full-scale trial. In the full-scale RCT, 524 participants will be recruited.
Discussion: The SPIN-SELF Program may improve disease management self-efficacy, patient activation, social appearance anxiety, and functional health outcomes in people with SSc. SPIN works with partner patient organizations around the world to disseminate its programs free-of-charge.
Trial registration: ClinicalTrials.gov NCT04246528 . Registered on 27 January 2020.
Keywords: Patient activation; Randomized controlled trial; Scleroderma; Self-efficacy; Self-management; Systemic sclerosis; e-Health.
© 2021. The Author(s).
Conflict of interest statement
All authors declare that they have no competing interests.
Figures




References
-
- EURORDIS Rare Diseases Europe. [http://www.eurordis.org/content/what-rare-disease] Accessed 10 June 2021.
-
- Mayes MD. Systemic sclerosis: clinical features. In: Klippel JH, Stone JH, Crofford LJ, White PH, editors. Primer on the Rheumatic Diseases. 13. New York: Springer and Arthritis Foundation; 2008. pp. 343–350.
-
- Thombs BD, van Lankveld W, Bassel M, Baron M, Buzza R, Haslam S, Haythornthwaite JA, Hudson M, Jewett LR, Knafo R, Kwakkenbos L, Malcarne VL, Milette K, Motivala SJ, Newton EG, Nielson WR, Pacy M, Razykov I, Schieir O, Taillefer S, Worron-Sauve M. Psychological health and well-being in systemic sclerosis: State of the science and consensus research agenda. Arthritis Care Res. 2010;62(8):1181–1189. doi: 10.1002/acr.20187. - DOI - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

