Power calculator for detecting allelic imbalance using hierarchical Bayesian model
- PMID: 34838135
- PMCID: PMC8626927
- DOI: 10.1186/s13104-021-05851-x
Power calculator for detecting allelic imbalance using hierarchical Bayesian model
Abstract
Objective: Allelic imbalance (AI) is the differential expression of the two alleles in a diploid. AI can vary between tissues, treatments, and environments. Methods for testing AI exist, but methods are needed to estimate type I error and power for detecting AI and difference of AI between conditions. As the costs of the technology plummet, what is more important: reads or replicates?
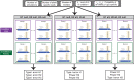
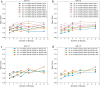
Results: We find that a minimum of 2400, 480, and 240 allele specific reads divided equally among 12, 5, and 3 replicates is needed to detect a 10, 20, and 30%, respectively, deviation from allelic balance in a condition with power > 80%. A minimum of 960 and 240 allele specific reads divided equally among 8 replicates is needed to detect a 20 or 30% difference in AI between conditions with comparable power. Higher numbers of replicates increase power more than adding coverage without affecting type I error. We provide a Python package that enables simulation of AI scenarios and enables individuals to estimate type I error and power in detecting AI and differences in AI between conditions.
Keywords: Allele specific reads; Allelic imbalance; Biological replicates; Power; Simulation; Type I error.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Direct Testing for Allele-Specific Expression Differences Between Conditions.G3 (Bethesda). 2018 Feb 2;8(2):447-460. doi: 10.1534/g3.117.300139. G3 (Bethesda). 2018. PMID: 29167272 Free PMC article.
-
Airpart: interpretable statistical models for analyzing allelic imbalance in single-cell datasets.Bioinformatics. 2022 May 13;38(10):2773-2780. doi: 10.1093/bioinformatics/btac212. Bioinformatics. 2022. PMID: 35561168 Free PMC article.
-
Testcrosses are an efficient strategy for identifying cis-regulatory variation: Bayesian analysis of allele-specific expression (BayesASE).G3 (Bethesda). 2021 May 7;11(5):jkab096. doi: 10.1093/g3journal/jkab096. G3 (Bethesda). 2021. PMID: 33772539 Free PMC article.
-
A flexible Bayesian method for detecting allelic imbalance in RNA-seq data.BMC Genomics. 2014 Oct 23;15(1):920. doi: 10.1186/1471-2164-15-920. BMC Genomics. 2014. PMID: 25339465 Free PMC article.
-
Perspectives on Allele-Specific Expression.Annu Rev Biomed Data Sci. 2021 Jul 20;4:101-122. doi: 10.1146/annurev-biodatasci-021621-122219. Epub 2021 Apr 28. Annu Rev Biomed Data Sci. 2021. PMID: 34465174 Review.
Cited by
-
Denervation alters the secretome of myofibers and thereby affects muscle stem cell lineage progression and functionality.NPJ Regen Med. 2024 Mar 1;9(1):10. doi: 10.1038/s41536-024-00353-3. NPJ Regen Med. 2024. PMID: 38424446 Free PMC article.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

