Effectiveness of ChAdOx1 nCoV-19 vaccine against SARS-CoV-2 infection during the delta (B.1.617.2) variant surge in India: a test-negative, case-control study and a mechanistic study of post-vaccination immune responses
- PMID: 34838183
- PMCID: PMC8616567
- DOI: 10.1016/S1473-3099(21)00680-0
Effectiveness of ChAdOx1 nCoV-19 vaccine against SARS-CoV-2 infection during the delta (B.1.617.2) variant surge in India: a test-negative, case-control study and a mechanistic study of post-vaccination immune responses
Abstract
Background: SARS-CoV-2 variants of concern (VOCs) have threatened COVID-19 vaccine effectiveness. We aimed to assess the effectiveness of the ChAdOx1 nCoV-19 vaccine, predominantly against the delta (B.1.617.2) variant, in addition to the cellular immune response to vaccination.
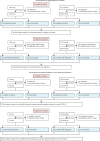
Methods: We did a test-negative, case-control study at two medical research centres in Faridabad, India. All individuals who had a positive RT-PCR test for SARS-CoV-2 infection between April 1, 2021, and May 31, 2021, were included as cases and individuals who had a negative RT-PCR test were included as controls after matching with cases on calendar week of RT-PCR test. The primary outcome was effectiveness of complete vaccination with the ChAdOx1 nCoV-19 vaccine against laboratory-confirmed SARS-CoV-2 infection. The secondary outcomes were effectiveness of a single dose against SARS-CoV-2 infection and effectiveness of a single dose and complete vaccination against moderate-to-severe disease among infected individuals. Additionally, we tested in-vitro live-virus neutralisation and T-cell immune responses to the spike protein of the wild-type SARS-CoV-2 and VOCs among healthy (anti-nucleocapsid antibody negative) recipients of the ChAdOx1 nCoV-19 vaccine.
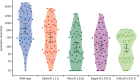
Findings: Of 2379 cases of confirmed SARS-CoV-2 infection, 85 (3·6%) were fully vaccinated compared with 168 (8·5%) of 1981 controls (adjusted OR [aOR] 0·37 [95% CI 0·28-0·48]), giving a vaccine effectiveness against SARS-CoV-2 infection of 63·1% (95% CI 51·5-72·1). 157 (6·4%) of 2451 of cases and 181 (9·1%) of 1994) controls had received a single dose of the ChAdOx1 nCoV-19 vaccine (aOR 0·54 [95% CI 0·42-0·68]), thus vaccine effectiveness of a single dose against SARS-CoV-2 infection was 46·2% (95% CI 31·6-57·7). One of 84 cases with moderate-to-severe COVID-19 was fully vaccinated compared with 84 of 2295 cases with mild COVID-19 (aOR 0·19 [95% CI 0·01-0·90]), giving a vaccine effectiveness of complete vaccination against moderate-to-severe disease of 81·5% (95% CI 9·9-99·0). The effectiveness of a single dose against moderate-to-severe disease was 79·2% (95% CI 46·1-94·0); four of 87 individuals with moderate-to-severe COVID-19 had received a single dose compared with 153 of 2364 participants with mild disease (aOR 0·20 [95% CI 0·06-0·54]). Among 49 healthy, fully vaccinated individuals, neutralising antibody responses were lower against the alpha (B.1.1.7; geometric mean titre 244·7 [95% CI 151·8-394·4]), beta (B.1.351; 97·6 [61·2-155·8]), kappa (B.1.617.1; 112·8 [72·7-175·0]), and delta (88·4 [61·2-127·8]) variants than against wild-type SARS-CoV-2 (599·4 [376·9-953·2]). However, the antigen-specific CD4 and CD8 T-cell responses were conserved against both the delta variant and wild-type SARS-CoV-2.
Interpretation: The ChAdOx1 nCoV-19 vaccine remained effective against moderate-to-severe COVID-19, even during a surge that was dominated by the highly transmissible delta variant of SARS-CoV-2. Spike-specific T-cell responses were maintained against the delta variant. Such cellular immune protection might compensate for waning humoral immunity.
Funding: Department of Biotechnology India, Council of Scientific and Industrial Research India, and Fondation Botnar.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests We declare no competing interests.
Figures

Comment in
-
COVID-19 vaccines in the age of the delta variant.Lancet Infect Dis. 2022 Apr;22(4):429-430. doi: 10.1016/S1473-3099(21)00688-5. Epub 2021 Nov 25. Lancet Infect Dis. 2022. PMID: 34838184 Free PMC article. No abstract available.
-
Effectiveness of ChAdOx1 nCoV-19 vaccine during the delta (B.1.617.2) variant surge in India.Lancet Infect Dis. 2022 Apr;22(4):446-447. doi: 10.1016/S1473-3099(22)00132-3. Lancet Infect Dis. 2022. PMID: 35338863 Free PMC article. No abstract available.
-
Effectiveness of ChAdOx1 nCoV-19 vaccine during the delta (B.1.617.2) variant surge in India - Authors' reply.Lancet Infect Dis. 2022 Apr;22(4):447. doi: 10.1016/S1473-3099(22)00120-7. Lancet Infect Dis. 2022. PMID: 35338864 Free PMC article. No abstract available.
References
-
- Public Health England SARS-CoV-2 variants of concern and variants under investigation. Technical briefing 10. https://assets.publishing.service.gov.uk/government/uploads/system/uploa...
-
- WHO COVID-19 weekly epidemiological update. https://www.who.int/docs/default-source/coronaviruse/situation-reports/2... - PMC - PubMed
-
- Wang P, Nair MS, Liu L, et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature. 2021;593:130–135. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

