Hydroxychloroquine and azithromycin used alone or combined are not effective against SARS-CoV-2 ex vivo and in a hamster model
- PMID: 34838583
- PMCID: PMC8611861
- DOI: 10.1016/j.antiviral.2021.105212
Hydroxychloroquine and azithromycin used alone or combined are not effective against SARS-CoV-2 ex vivo and in a hamster model
Abstract
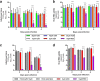
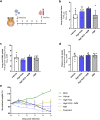
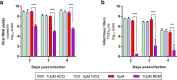
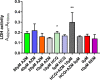
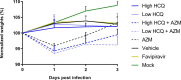
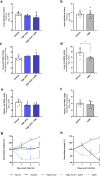
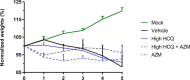
Drug repositioning has been used extensively since the beginning of the COVID-19 pandemic in an attempt to identify antiviral molecules for use in human therapeutics. Hydroxychloroquine and azithromycin have shown inhibitory activity against SARS-CoV-2 replication in different cell lines. Based on such in vitro data and despite the weakness of preclinical assessment, many clinical trials were set up using these molecules. In the present study, we show that hydroxychloroquine and azithromycin alone or combined does not block SARS-CoV-2 replication in human bronchial airway epithelia. When tested in a Syrian hamster model, hydroxychloroquine and azithromycin administrated alone or combined displayed no significant effect on viral replication, clinical course of the disease and lung impairments, even at high doses. Hydroxychloroquine quantification in lung tissues confirmed strong exposure to the drug, above in vitro inhibitory concentrations. Overall, this study does not support the use of hydroxychloroquine and azithromycin as antiviral drugs for the treatment of SARS-CoV-2 infections.
Keywords: Antivirals; Azithromycin; COVID-19; Coronavirus; Ex vivo; Human airway epithelium; Hydroxychloroquine; In vivo; SARS-CoV-2; Syrian hamster.
Copyright © 2021 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Barnard D.L., Day C.W., Bailey K., Heiner M., Montgomery R., Lauridsen L., Chan P.K., Sidwell R.W. Evaluation of immunomodulators, interferons and known in vitro SARS-coV inhibitors for inhibition of SARS-coV replication in BALB/c mice. Antivir. Chem. Chemother. 2006;17:275–284. - PubMed
-
- Baronas E.T., Lee J.-W., Alden C., Hsieh F.Y. Biomarkers to monitor drug-induced phospholipidosis. Toxicol. Appl. Pharmacol. 2007;218:72–78. - PubMed
-
- Browning D.J. Springer New York; New York, NY: 2014. Pharmacology of Chloroquine and Hydroxychloroquine, Hydroxychloroquine and Chloroquine Retinopathy; pp. 35–63.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

