Persistent sexually dimorphic effects of adolescent THC exposure on hippocampal synaptic plasticity and episodic memory in rodents
- PMID: 34838664
- PMCID: PMC10249649
- DOI: 10.1016/j.nbd.2021.105565
Persistent sexually dimorphic effects of adolescent THC exposure on hippocampal synaptic plasticity and episodic memory in rodents
Abstract
There is evidence that cannabis use during adolescence leads to memory and cognitive problems in young adulthood but little is known about effects of early life cannabis exposure on synaptic operations that are critical for encoding and organizing information. We report here that a 14-day course of daily Δ9-tetrahydrocannabinol treatments administered to adolescent rats and mice (aTHC) leads to profound but selective deficits in synaptic plasticity in two axonal systems in female, and to lesser extent male, hippocampus as assessed in adulthood. Adolescent-THC exposure did not alter basic synaptic transmission (input/output curves) and had only modest effects on frequency facilitation. Nevertheless, aTHC severely impaired the endocannabinoid-dependent long-term potentiation in the lateral perforant path in females of both species, and in male mice; this was reliably associated with impaired acquisition of a component of episodic memory that depends on lateral perforant path function. Potentiation in the Schaffer-commissural (S-C) projection to field CA1 was disrupted by aTHC treatment in females only and this was associated with both a deficit in estrogen effects on S-C synaptic responses and impairments to CA1-dependent spatial (object location) memory. In all the results demonstrate sexually dimorphic and projection system-specific effects of aTHC exposure that could underlie discrete effects of early life cannabinoid usage on adult cognitive function. Moreover they suggest that some of the enduring, sexually dimorphic effects of cannabis use reflect changes in synaptic estrogen action.
Keywords: CA1; Cannabinoid; Estrogen; Frequency facilitation; Lateral perforant path; Long-term potentiation; Sex differences; Spatial learning; THC; hippocampus.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest
The authors declare no competing interests.
Figures


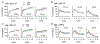
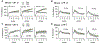




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

