Immunogenicity trends 1 and 3 months after second BNT162B2 vaccination among healthcare workers in Israel
- PMID: 34838782
- PMCID: PMC8611821
- DOI: 10.1016/j.cmi.2021.11.014
Immunogenicity trends 1 and 3 months after second BNT162B2 vaccination among healthcare workers in Israel
Abstract
Objectives: We evaluated the antibody response to the BNT162B2 vaccine among healthcare workers (HCWs) to identify factors associated with decreased immunogenicity.
Methods: This prospective cohort study included consenting HCWs who completed a questionnaire regarding background illnesses, medications, and post-vaccination allergic reactions or rash. All HCWs were tested for anti-spike antibodies (LIAISON SARS-CoV-2 S1/S2 IgG assay) 1 and 3 months after the second vaccine dose. A multivariate mixed linear model was adjusted to participants' data and fit to predict antibody levels after the second BNT162B2 vaccine dose, based on antibody levels at 1 month and the slope between 3 months and 1 month. Multivariate analyses identified factors associated with lower antibody levels.
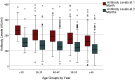
Results: In total 1506 HCWs were tested for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies. Older age was associated with lower mean antibody levels (-1.22 AU/mL, p < 0.001, 95%CI -1.43 to -1.01). In addition, male sex (-22.16 AU/mL, p < 0.001, 95%CI -27.93 to -16.39), underlying condition (-10.86 AU/mL, p 0.007, 95%CI -18.81 to -2.91) and immunosuppressive treatment (-28.57 AU/mL, p 0.002, 95%CI -46.85 to -10.29) were associated with significantly lower mean antibody levels. Allergic reactions after vaccine administration or peri-vaccination glucocorticosteroid treatment were not correlated with antibody levels.
Conclusions: Most HCWs had measurable antibodies at 3 months. Risk factors for lower antibody levels were older age, male sex, underlying condition, and immunosuppressive treatment. These factors may be considered when planning booster doses during vaccine shortages.
Keywords: BNT162b2; COVID-19; Healthcare workers; Immunogenicity; Serology.
Copyright © 2021 European Society of Clinical Microbiology and Infectious Diseases. Published by Elsevier Ltd. All rights reserved.
Figures
References
-
- World Health Organization (WHO) WHO; Geneva: 2021. WHO coronavirus disease (COVID-19) dashboard.https://covid19.who.int/ Available from:
-
- Ministry of Health of Israel . Ministry of Health; Jerusalem, Israel: 2021. Coronavirus in Israel—general situation.https://datadashboard.health.gov.il/COVID-19/general Hebrew. Available from:
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous