Pharmacoresponsiveness of spontaneous recurrent seizures and the comorbid sleep disorder of epileptic Kcna1-null mice
- PMID: 34838797
- PMCID: PMC8787849
- DOI: 10.1016/j.ejphar.2021.174656
Pharmacoresponsiveness of spontaneous recurrent seizures and the comorbid sleep disorder of epileptic Kcna1-null mice
Abstract
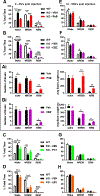
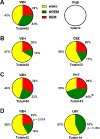
Drug resistant epilepsy affects ∼30% of people with epilepsy and is associated with epilepsy syndromes with frequent and multiple types of seizures, lesions or cytoarchitectural abnormalities, increased risk of mortality and comorbidities such as cognitive impairment and sleep disorders. A limitation of current preclinical models is that spontaneous seizures with comorbidities take time to induce and test, thus making them low-throughput. Kcna1-null mice exhibit all the characteristics of drug resistant epilepsy with spontaneous seizures and comorbidities occurring naturally; thus, we aimed to determine whether they also demonstrate pharmacoresistanct seizures and the impact of medications on their sleep disorder comorbidity. In this exploratory study, Kcna1-null mice were treated with one of four conventional antiseizure medications, carbamazepine, levetiracetam, phenytoin, and phenobarbital using a moderate throughput protocol (vehicle for 2 days followed by 2 days of treatment with high therapeutic doses selected based on published data in the 6 Hz model of pharmacoresistant seizures). Spontaneous recurrent seizures and vigilance states were recorded with video-EEG/EMG. Carbamazepine, levetiracetam and phenytoin had partial efficacy (67%, 75% and 33% were seizure free, respectively), whereas phenobarbital was fully efficacious and conferred seizure freedom to all mice. Thus, seizures of Kcna1-null mice appear to be resistant to three of the drugs tested. Levetiracetam failed to affect sleep architecture, carbamazepine and phenytoin had moderate effects, and phenobarbital, as predicted, restored sleep architecture. Data suggest Kcna1-null mice may be a moderate throughput model of drug resistant epilepsy useful in determining mechanisms of pharmacoresistance and testing novel therapeutic strategies.
Keywords: Carbamazepine; Carbamazepine (PubChem CID: 2554); Chemical compounds studied in this article are phenobarbital (PubChem CID: 4763); Diurnal; Kv1.1; Levetiracetam; Levetiracetam (PubChem CID: 5284583); Phenobarbital; Phenytoin; Phenytoin (PubChem CID: 1775).
Copyright © 2021. Published by Elsevier B.V.
Conflict of interest statement
Declaration of Interest
None of the authors has any conflict of interest to disclose.
Figures




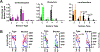
Similar articles
-
Orexin Receptor Antagonism Improves Sleep and Reduces Seizures in Kcna1-null Mice.Sleep. 2016 Feb 1;39(2):357-68. doi: 10.5665/sleep.5444. Sleep. 2016. PMID: 26446112 Free PMC article.
-
Spontaneous recurrent seizures in an intra-amygdala kainate microinjection model of temporal lobe epilepsy are differentially sensitive to antiseizure drugs.Exp Neurol. 2022 Mar;349:113954. doi: 10.1016/j.expneurol.2021.113954. Epub 2021 Dec 17. Exp Neurol. 2022. PMID: 34922908 Free PMC article.
-
Pharmacogenetics of KCNQ channel activation in 2 potassium channelopathy mouse models of epilepsy.Epilepsia. 2018 Feb;59(2):358-368. doi: 10.1111/epi.13978. Epub 2017 Dec 19. Epilepsia. 2018. PMID: 29265344 Free PMC article.
-
Animal Models of Seizures and Epilepsy: Past, Present, and Future Role for the Discovery of Antiseizure Drugs.Neurochem Res. 2017 Jul;42(7):1873-1888. doi: 10.1007/s11064-017-2222-z. Epub 2017 Mar 13. Neurochem Res. 2017. PMID: 28290134 Review.
-
Antiseizure Medications for Adults With Epilepsy: A Review.JAMA. 2022 Apr 5;327(13):1269-1281. doi: 10.1001/jama.2022.3880. JAMA. 2022. PMID: 35380580 Review.
Cited by
-
Metabolic aspects of genetic ion channel epilepsies.J Neurochem. 2024 Dec;168(12):3911-3935. doi: 10.1111/jnc.15938. Epub 2023 Aug 18. J Neurochem. 2024. PMID: 37594756 Free PMC article. Review.
-
Sudden Unexpected Death in Epilepsy: A Narrative Review of Mechanism, Risks, and Prevention.J Clin Med. 2025 May 10;14(10):3329. doi: 10.3390/jcm14103329. J Clin Med. 2025. PMID: 40429323 Free PMC article. Review.
-
Sudden unexpected death in epilepsy.Curr Opin Neurol. 2022 Apr 1;35(2):181-188. doi: 10.1097/WCO.0000000000001034. Curr Opin Neurol. 2022. PMID: 35102124 Free PMC article. Review.
-
SUDEP: A Local Low Pressure [pO2] System Makes It Hard to Breathe.Epilepsy Curr. 2023 Jul 19;23(5):327-329. doi: 10.1177/15357597231186914. eCollection 2023 Sep-Oct. Epilepsy Curr. 2023. PMID: 37901771 Free PMC article. No abstract available.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

