Harnessing galactose oxidase in the development of a chemoenzymatic platform for glycoconjugate vaccine design
- PMID: 34838818
- PMCID: PMC8689215
- DOI: 10.1016/j.jbc.2021.101453
Harnessing galactose oxidase in the development of a chemoenzymatic platform for glycoconjugate vaccine design
Abstract
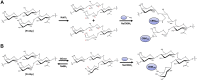
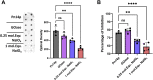
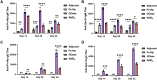
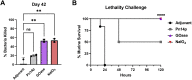
In the preparation of commercial conjugate vaccines, capsular polysaccharides (CPSs) must undergo chemical modification to generate the reactive groups necessary for covalent attachment to a protein carrier. One of the most common approaches employed for this derivatization is sodium periodate (NaIO4) oxidation of vicinal diols found within CPS structures. This procedure is largely random and structurally damaging, potentially resulting in significant changes in the CPS structure and therefore its antigenicity. Additionally, periodate activation of CPS often gives rise to heterogeneous conjugate vaccine products with variable efficacy. Here, we explore the use of an alternative agent, galactose oxidase (GOase) isolated from Fusarium sp. in a chemoenzymatic approach to generate a conjugate vaccine against Streptococcus pneumoniae. Using a colorimetric assay and NMR spectroscopy, we found that GOase generated aldehyde motifs on the CPS of S. pneumoniae serotype 14 (Pn14p) in a site-specific and reversible fashion. Direct comparison of Pn14p derivatized by either GOase or NaIO4 illustrates the functionally deleterious role chemical oxidation can have on CPS structures. Immunization with the conjugate synthesized using GOase provided a markedly improved humoral response over the traditional periodate-oxidized group. Further, functional protection was validated in vitro by measure of opsonophagocytic killing and in vivo through a lethality challenge in mice. Overall, this work introduces a strategy for glycoconjugate development that overcomes limitations previously known to play a role in the current approach of vaccine design.
Keywords: Streptococcus pneumoniae; capsular polysaccharide; galactose oxidase; glycoconjugate vaccine; pneumonia; vaccine development.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare no conflict of interest with the contents of this article.
Figures

Comment in
-
Expanding polysaccharide-protein coupling of glycoconjugate vaccines.J Biol Chem. 2022 Mar;298(3):101755. doi: 10.1016/j.jbc.2022.101755. Epub 2022 Feb 22. J Biol Chem. 2022. PMID: 35202656 Free PMC article.
References
-
- Kadioglu A., Weiser J.N., Paton J.C., Andrew P.W. The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat. Rev. Microbiol. 2008;6:288–301. - PubMed
-
- Avci F.Y., Kasper D.L. How bacterial carbohydrates influence the adaptive immune system. Annu. Rev. Immunol. 2009;28:107–130. - PubMed
-
- Stein K.E. Thymus-independent and thymus-dependent responses to polysaccharide antigens. J. Infect. Dis. 1992;165:S49–S52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

