Effect of Magnesium on Dentinogenesis of Human Dental Pulp Cells
- PMID: 34840576
- PMCID: PMC8616686
- DOI: 10.1155/2021/6567455
Effect of Magnesium on Dentinogenesis of Human Dental Pulp Cells
Abstract
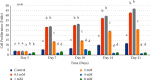
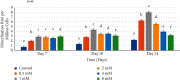
Introducing therapeutic ions into pulp capping materials has been considered a new approach for enhancing regeneration of dental tissues. However, no studies have been reported on its dentinogenic effects on human dental pulp cells (HDPCs). This study was designed to investigate the effects of magnesium (Mg2+) on cell attachment efficiency, proliferation, differentiation, and mineralization of HDPCs. HDPCs were cultured with 0.5 mM, 1 mM, 2 mM, 4 mM, and 8 mM concentrations of supplemental Mg2+ and 0 mM (control). Cell attachment was measured at 4, 8, 12, 16, and 20 hours. Cell proliferation rate was evaluated at 3, 7, 10, 14, and 21 days. Crystal violet staining was used to determine cell attachment and proliferation rate. Alkaline phosphatase (ALP) activity was assessed using the fluorometric assay at 7, 10, and 14 days. Mineralization of cultures was measured by Alizarin red staining. Statistical analysis was done using multiway analysis of variance (multiway ANOVA) with Wilks' lambda test. Higher cell attachment was shown with 0.5 mM and 1 mM at 16 hours compared to control (P < 0.0001). Cells with 0.5 mM and 1 mM supplemental Mg2+ showed significantly higher proliferation rates than control at 7, 10, 14, and 21 days (P < 0.0001). However, cell proliferation rates decreased significantly with 4 mM and 8 mM supplemental Mg2+ at 14 and 21 days (P < 0.0001). Significantly higher levels of ALP activity and mineralization were observed in 0.5 mM, 1 mM, and 2 mM supplemental Mg2+ at 10 and 14 days (P < 0.0001). However, 8 mM supplemental Mg2+ showed lower ALP activity compared to control at 14 days (P < 0.0001), while 4 mM and 8 mM supplemental Mg2+showed less mineralization compared to control (P < 0.0001). The study indicated that the optimal (0.5-2 mM) supplemental Mg2+ concentrations significantly upregulated HDPCs by enhancing cell attachment, proliferation rate, ALP activity, and mineralization. Magnesium-containing biomaterials could be considered for a future novel dental pulp-capping additive in regenerative endodontics.
Copyright © 2021 Rania M. Salem et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures



References
-
- NICDR. Dental caries (tooth decay) in adults (age 20 to 64) 2014. http://www.nidcr.nih.gov.ezproxy.bu.edu/DataStatistics/FindDataByTopic/D... .
LinkOut - more resources
Full Text Sources

