Evaluating the Effect of Incobotulinumtoxin A for Glabellar, Forehead, and Crow's Feet Lines Using A High Dilution
- PMID: 34840655
- PMCID: PMC8570655
Evaluating the Effect of Incobotulinumtoxin A for Glabellar, Forehead, and Crow's Feet Lines Using A High Dilution
Abstract
Background: As aesthetic preferences have evolved and patients wish their muscles to be relaxed, but not frozen, a higher dilution of incobotulinumtoxinA (INCO) has allowed for increased spread using fewer units, yet no studies to date have investigated the efficacy, longevity, and safety of hyperdiluted INCO.
Objective: We evaluated the effect of incobotulinumtoxinA (INCO) in glabellar, forehead, and lateral periorbital lines using a high dilution.
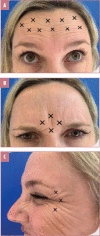
Methods: Subjects with moderate-to-severe upper facial lines at rest according to the Merz Aesthetics Scales™ (Merz Pharmaceuticals GmbH, Frankfurt am Main, Germany) received 15U of INCO to the glabellar (n=4 injection sites), 10U to the rest of the forehead (n=10 injection sites), and 5U to the lateral periorbital lines (n=3 injection sites/eye). Primary outcomes were physician- and subject-rated improvement at one month using the Global Aesthetic Improvement Scale (GAIS) and changes in line severity using the Merz Aesthetics Scales™.
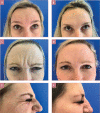
Results: The study included 15 women aged 35 to 65 years. At one month, physician GAIS scores indicated 91.2% of subjects were very much improved and 8.8% were much improved; 91.5%, 78.0%, and 57.6% of participants remained at least improved at four, five, and six months, respectively. Subject GAIS scores at one month were in agreement with physician scores. At one month, an improvement of at least one point in Merz Aesthetics Scales™ scores in glabellar, forehead, and lateral periorbital lines was reported in 88.9%, 98.3%, and 94.8% of participants, respectively. Subject satisfaction was high throughout the study. No treatment-related adverse events were observed.
Conclusion: Hyperdilute INCO was effective at improving overall appearance and reducing line severity in individuals with moderate-to-severe upper facial lines. Patient satisfaction was maintained up to six months and treatment was well tolerated.
Keywords: IncobotulinumtoxinA; glabellar lines; horizontal forehead lines; hyperdiluted; lateral periorbital lines.
Copyright © 2021. Matrix Medical Communications. All rights reserved.
Conflict of interest statement
DISCLOSURES: Dr. Barbarino is a consultant for Merz, Lumenis, Alastin Skincare, Skinceuticals, Syneron/Candela, and Sinclair Pharmaceuticals. Dr. Burgess is a consultant for Merz and Allergan. Dr. van Loghem is a consultant for Merz, Allergan, and Ipsen. Dr. Corduff is a clinical advisor, investigator, and speaker for Merz and medical advisory board member for Establishment Labs.
Figures




References
-
- International Society of Aesthetic Plastic Surgery (ISAPS). ISAPS international survey on aesthetic/cosmetic procedures performed in 2018. https://www.isaps.org/wp-content/uploads/2019/12/ISAPS-Global-Survey-Res... Available at: Accessed June 2, 2020. - PubMed
-
- The Aesthetic Society. Aesthetic Plastic Surgery National Databank Statistics 2019. https://www.surgery.org/media/statistics Available at: Accessed June 2, 2020.
-
- Kaminer MS, Cox SE, Fagien S et al. Re-examining the optimal use of neuromodulators and the changing landscape: a Consensus panel update. J Drugs Dermatol. 2020;19(4):s5–s15. - PubMed
-
- Kerscher M, Wanitphakdeedecha R, Trindade de Almeida A et al. IncobotulinumtoxinA: a highly purified and precisely manufactured botulinum neurotoxin type A. J Drugs Dermatol. 2019;18(1):52–57. - PubMed
-
- Xeomin prescribing information. XEOMIN—incobotulinumtoxina injection, powder, lyophilized, for solution. 2019 Merz Pharmaceuticals, LLC. https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=ccdc3aae-... Available at: Accessed June 2, 2020.
LinkOut - more resources
Full Text Sources
Research Materials
