Optogenetic axon guidance in embryonic zebrafish
- PMID: 34841275
- PMCID: PMC8605397
- DOI: 10.1016/j.xpro.2021.100947
Optogenetic axon guidance in embryonic zebrafish
Abstract
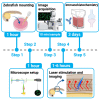
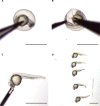
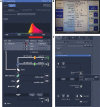
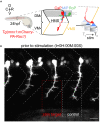
Axons form the long-range connections of biological neuronal networks, which are built through the developmental process of axon guidance. Here, we describe a protocol to precisely and non-invasively control axonal growth trajectories in live zebrafish embryos using focal light activation of a photoactivatable Rac1. We outline techniques for photostimulation, time-lapse imaging, and immunohistochemistry. These approaches enable engineering of long-range axonal circuitry or repair of defective circuits in living zebrafish, despite a milieu of competing endogenous signals and repulsive barriers. For complete details on the use and execution of this protocol, please refer to Harris et al. (2020).
Keywords: Developmental biology; Microscopy; Model Organisms; Molecular Biology; Neuroscience.
© 2021.
Conflict of interest statement
P.A. is a consultant for Herophilus, Foresite Labs, and the New York Stem Cell Foundation. P.A. is a co-founder of Serqet Therapeutics. A patent application is pending based on this work (applicants: President and Fellows of Harvard College; inventors: P.A. and J.M.H). All other authors declare no competing interests.
Figures



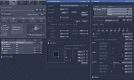
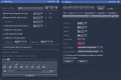
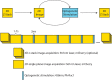

References
-
- Beattie C.E. Control of motor axon guidance in the zebrafish embryo. Brain Res. Bull. 2000;53:489–500. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

