Electronic health record-based genome-wide meta-analysis provides insights on the genetic architecture of non-alcoholic fatty liver disease
- PMID: 34841290
- PMCID: PMC8606899
- DOI: 10.1016/j.xcrm.2021.100437
Electronic health record-based genome-wide meta-analysis provides insights on the genetic architecture of non-alcoholic fatty liver disease
Abstract
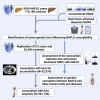
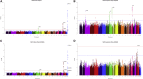
Non-alcoholic fatty liver disease (NAFLD) is a complex disease linked to several chronic diseases. We aimed at identifying genetic variants associated with NAFLD and evaluating their functional consequences. We performed a genome-wide meta-analysis of 4 cohorts of electronic health record-documented NAFLD in participants of European ancestry (8,434 cases and 770,180 controls). We identify 5 potential susceptibility loci for NAFLD (located at or near GCKR, TR1B1, MAU2/TM6SF2, APOE, and PNPLA3). We also report a potentially causal effect of lower LPL expression in adipose tissue on NAFLD susceptibility and an effect of the FTO genotype on NAFLD. Positive genetic correlations between NAFLD and cardiometabolic diseases and risk factors such as body fat accumulation/distribution, lipoprotein-lipid levels, insulin resistance, and coronary artery disease and negative genetic correlations with parental lifespan, socio-economic status, and acetoacetate levels are observed. This large GWAS meta-analysis reveals insights into the genetic architecture of NAFLD.
Keywords: adipose tissue; electronic health records; genetics; genome-wide association study; lipoprotein lipase; non-alcoholic fatty liver disease.
© 2021 The Author(s).
Conflict of interest statement
A.T. receives research funding from Johnson & Johnson Medical Companies, Medtronic, Bodynov, and GI Windows for studies on bariatric surgery and has received consulting fees from Novo Nordisk and Bausch Health. A.V.K. has served as a scientific advisor to Sanofi, Amgen, Maze Therapeutics, Navitor Pharmaceuticals, Sarepta Therapeutics, Novartis, Verve Therapeutics, Silence Therapeutics, Veritas International, Color Health, Third Rock Ventures, and Columbia University (NIH); has received speaking fees from Illumina, MedGenome, Amgen, and the Novartis Institute for Biomedical Research; and has received sponsored research agreements from the Novartis Institute for Biomedical Research and IBM Research. B.J.A. is a consultant for Novartis and Silence Therapeutics and has received research contracts from Pfizer, Ionis Pharmaceuticals, and Silence Therapeutics.
Figures


References
-
- Stefan N., Häring H.-U., Cusi K. Non-alcoholic fatty liver disease: causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol. 2019;7:313–324. - PubMed
-
- Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. - PubMed
-
- Eguchi Y., Hyogo H., Ono M., Mizuta T., Ono N., Fujimoto K., Chayama K., Saibara T., JSG-NAFLD Prevalence and associated metabolic factors of nonalcoholic fatty liver disease in the general population from 2009 to 2010 in Japan: a multicenter large retrospective study. J. Gastroenterol. 2012;47:586–595. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

