Heat shock enhances outer-membrane vesicle release in Bordetella spp
- PMID: 34841303
- PMCID: PMC8610307
- DOI: 10.1016/j.crmicr.2020.100009
Heat shock enhances outer-membrane vesicle release in Bordetella spp
Abstract
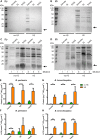
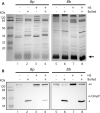
Pertussis, also known as whooping cough, is caused by the Gram-negative bacterium Bordetella pertussis, an obligate human pathogen. Despite high vaccination rates in high-income countries, resurgence of pertussis cases is an occurring problem that urges the necessity of developing an improved vaccine. Likewise, the efficacy of vaccines for Bordetella bronchiseptica, which causes similar disease in pigs and companion animals, is debatable. A promising approach for novel vaccines is the use of outer membrane vesicles (OMVs). However, spontaneous OMV (sOMV) release by Bordetella spp. is too low for cost-effective vaccine production. Therefore, we investigated the influence of growth in various media commonly used for culturing Bordetella in the Bvg+, i.e. virulent, phase and of a heat shock applied to inactivate the cells on OMV production. Inactivation of the bacterial cells at 56 °C before OMV isolation greatly enhanced OMV release in both Bordetella spp. without causing significant cell lysis. The growth medium used barely affected the efficiency of OMV release but did affect the protein pattern of the OMVs. Differences were found to be related, at least in part, to different availability of the nutrient metals iron and zinc in the media and include expression of potentially relevant vaccine antigens, such as the receptors FauA and ZnuD. The protein content of OMVs released by heat shock was comparable to that of sOMVs as determined by SDS-PAGE and Western blot analysis, and their heat-modifiable electrophoretic mobility suggests that also protein conformation is unaffected. However, significant differences were noticed between the protein content of OMVs and that of a purified outer membrane fraction, with two major outer membrane proteins, porin OmpP and the peptidoglycan-associated RmpM, being underrepresented in the OMVs. Altogether, these results indicate that the application of a heat shock is potentially an important step in the development of cost-effective, OMV-based vaccines for both Bordetella spp.
© 2020 The Authors.
Conflict of interest statement
Part of this work is included in a European patent application (EP20187477.3) with EFdJ, MDB, HPH, and JT as inventors. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





Similar articles
-
Comparison of Two Different Methods for the Extraction of Outer Membrane Vesicles from the Bordetella pertussis as a Vaccine Candidate.Arch Razi Inst. 2021 Sep 1;76(3):411-419. doi: 10.22092/ARI.2020.342861.1487. eCollection 2021 Summer. Arch Razi Inst. 2021. PMID: 34824734 Free PMC article.
-
Pal depletion results in hypervesiculation and affects cell morphology and outer-membrane lipid asymmetry in bordetellae.Res Microbiol. 2022 May-Jun;173(4-5):103937. doi: 10.1016/j.resmic.2022.103937. Epub 2022 Mar 3. Res Microbiol. 2022. PMID: 35248703
-
Outer Membrane Vesicles (OMV)-based and Proteomics-driven Antigen Selection Identifies Novel Factors Contributing to Bordetella pertussis Adhesion to Epithelial Cells.Mol Cell Proteomics. 2018 Feb;17(2):205-215. doi: 10.1074/mcp.RA117.000045. Epub 2017 Dec 4. Mol Cell Proteomics. 2018. PMID: 29203497 Free PMC article.
-
Bordetella pertussis and outer membrane vesicles.Pathog Glob Health. 2023 Jun;117(4):342-355. doi: 10.1080/20477724.2022.2117937. Epub 2022 Sep 1. Pathog Glob Health. 2023. PMID: 36047634 Free PMC article. Review.
-
Outer Membrane Vesicle Induction and Isolation for Vaccine Development.Front Microbiol. 2021 Feb 4;12:629090. doi: 10.3389/fmicb.2021.629090. eCollection 2021. Front Microbiol. 2021. PMID: 33613498 Free PMC article. Review.
Cited by
-
Reduction of endotoxicity in Bordetella bronchiseptica by lipid A engineering: Characterization of lpxL1 and pagP mutants.Virulence. 2021 Dec;12(1):1452-1468. doi: 10.1080/21505594.2021.1929037. Virulence. 2021. PMID: 34053396 Free PMC article.
-
Inactivation of the Mla system and outer-membrane phospholipase A results in disrupted outer-membrane lipid asymmetry and hypervesiculation in Bordetella pertussis.Curr Res Microb Sci. 2022 Nov 12;3:100172. doi: 10.1016/j.crmicr.2022.100172. eCollection 2022. Curr Res Microb Sci. 2022. PMID: 36518166 Free PMC article.
-
Biosynthesis of the Inner Core of Bordetella pertussis Lipopolysaccharides: Effect of Mutations on LPS Structure, Cell Division, and Toll-like Receptor 4 Activation.Int J Mol Sci. 2023 Dec 9;24(24):17313. doi: 10.3390/ijms242417313. Int J Mol Sci. 2023. PMID: 38139140 Free PMC article.
-
Outer Membrane Vesicles Coating Nano-Glycyrrhizic Acid Confers Protection Against Borderella bronchiseptica Through Th1/Th2/Th17 Responses.Int J Nanomedicine. 2022 Feb 11;17:647-663. doi: 10.2147/IJN.S350846. eCollection 2022. Int J Nanomedicine. 2022. PMID: 35177904 Free PMC article.
-
Bacterial Outer Membrane Vesicles and Immune Modulation of the Host.Membranes (Basel). 2023 Aug 24;13(9):752. doi: 10.3390/membranes13090752. Membranes (Basel). 2023. PMID: 37755174 Free PMC article. Review.
References
-
- Afonina G., Leduc I., Nepluev I., Jeter C., Routh P., Almond G., Orndorff P.E., Hobbs M., Elkins C. Immunization with the Haemophilus ducreyi hemoglobin receptor HgbA protects against infection in the swine model of chancroid. Infect. Immun. 2006;74:2224–2232. doi: 10.1128/IAI.74.4.2224-2232.2006. - DOI - PMC - PubMed
-
- Balhuizen M.D., Versluis C.M., van Harten R.M., de Jonge E.F., Brouwers J.F., van de Lest C.H.A., Veldhuizen E.J.A., Tommassen J., Haagsman H.P. PMAP-36 reduces the innate immune response induced by Bordetella bronchiseptica-derived outer membrane vesicles. Curr. Res. Microb. Sci. 2020 doi: 10.1016/j.crmicr.2020.100010. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

