Recent Advances and Prospects in Colloidal Nanomaterials
- PMID: 34841404
- PMCID: PMC8611664
- DOI: 10.1021/jacsau.1c00339
Recent Advances and Prospects in Colloidal Nanomaterials
Abstract
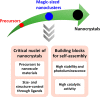
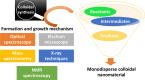
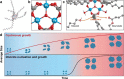
Colloidal nanomaterials of metals, metal oxides, and metal chalcogenides have attracted great attention in the past decade owing to their potential applications in optoelectronics, catalysis, and energy conversion. Introduction of various synthetic routes has resulted in diverse colloidal nanostructured materials with well-controlled size, shape, and composition, enabling the systematic study of their intriguing physicochemical, optoelectronic, and chemical properties. Furthermore, developments in the instrumentation have offered valuable insights into the nucleation and growth mechanism of these nanomaterials, which are crucial in designing prospective materials with desired properties. In this perspective, recent advances in the colloidal synthesis and mechanism studies of nanomaterials of metal chalcogenides, metals, and metal oxides are discussed. In addition, challenges in the characterization and future direction of the colloidal nanomaterials are provided.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Loiudice A.; Segura Lecina O.; Buonsanti R. Atomic Control in Multicomponent Nanomaterials: when Colloidal Chemistry Meets Atomic Layer Deposition. ACS Mater. Lett. 2020, 2, 1182–1202. 10.1021/acsmaterialslett.0c00271. - DOI
-
- Strach M.; Mantella V.; Pankhurst J. R.; Iyengar P.; Loiudice A.; Das S.; Corminboeuf C.; van Beek W.; Buonsanti R. Insights into Reaction Intermediates to Predict Synthetic Pathways for Shape-Controlled Metal Nanocrystals. J. Am. Chem. Soc. 2019, 141, 16312–16322. 10.1021/jacs.9b06267. - DOI - PubMed
-
- Huang J.; Buonsanti R. Colloidal Nanocrystals as Heterogeneous Catalysts for Electrochemical CO2 Conversion. Chem. Mater. 2019, 31, 13–25. 10.1021/acs.chemmater.8b04155. - DOI
-
- Lee J.; Yang J.; Kwon S. G.; Hyeon T. Nonclassical nucleation and growth of inorganic nanoparticles. Nat. Rev. Mater. 2016, 1, 16034.10.1038/natrevmats.2016.34. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
