Epicardial and endothelial cell activation concurs with extracellular matrix remodeling in atrial fibrillation
- PMID: 34841686
- PMCID: PMC8567047
- DOI: 10.1002/ctm2.558
Epicardial and endothelial cell activation concurs with extracellular matrix remodeling in atrial fibrillation
Abstract
Background: Improved understanding of the interconnectedness of structural remodeling processes in atrial fibrillation (AF) in patients could identify targets for future therapies.
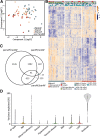
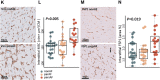
Methods: We present transcriptome sequencing of atrial tissues of patients without AF, with paroxysmal AF, and persistent AF (total n = 64). RNA expression levels were validated in the same and an independent cohort with qPCR. Biological processes were assessed with histological and immunohistochemical analyses.
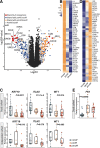
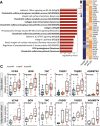
Results: In AF patients, epicardial cell gene expression decreased, contrasting with an upregulation of epithelial-to-mesenchymal transition (EMT) and mesenchymal cell gene expression. Immunohistochemistry demonstrated thickening of the epicardium and an increased proportion of (myo)fibroblast-like cells in the myocardium, supporting enhanced EMT in AF. We furthermore report an upregulation of endothelial cell proliferation, angiogenesis, and endothelial signaling. EMT and endothelial cell proliferation concurred with increased interstitial (myo)fibroblast-like cells and extracellular matrix gene expression including enhanced tenascin-C, thrombospondins, biglycan, and versican. Morphological analyses discovered increased and redistributed glycosaminoglycans and collagens in the atria of AF patients. Signaling pathways, including cell-matrix interactions, PI3K-AKT, and Notch signaling that could regulate mesenchymal cell activation, were upregulated.
Conclusion: Our results suggest that EMT and endothelial cell proliferation work in concert and characterize the (myo)fibroblast recruitment and ECM remodeling of AF. These processes could guide future research toward the discovery of targets for AF therapy.
Keywords: angiogenesis; arrhythmias; atrial fibrillation; atrial remodeling; epithelial-to-mesenchymal transition; extracellular matrix; transcriptome.
© 2021 The Authors. Clinical and Translational Medicine published by John Wiley & Sons Australia, Ltd on behalf of Shanghai Institute of Clinical Bioinformatics.
Figures





References
-
- Hindricks G, Potpara T, Dagres N, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio‐Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42(5):373‐498. - PubMed
-
- Schotten U, Neuberger H‐R, Allessie MA. The role of atrial dilatation in the domestication of atrial fibrillation. Prog Biophys Mol Biol. 2003;82(1‐3):151‐162. - PubMed
-
- Dzeshka MS, Lip GYH, Snezhitskiy V, Shantsila E. Cardiac fibrosis in patients with atrial fibrillation: mechanisms and clinical implications. J Am Coll Cardiol. 2015;66(8):943‐959. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
