99m Technetium-pyrophosphate scintigraphy: a practical guide for early diagnosis of transthyretin amyloid cardiomyopathy
- PMID: 34841715
- PMCID: PMC8788016
- DOI: 10.1002/ehf2.13693
99m Technetium-pyrophosphate scintigraphy: a practical guide for early diagnosis of transthyretin amyloid cardiomyopathy
Erratum in
-
Corrigendum.ESC Heart Fail. 2022 Aug;9(4):2764-2765. doi: 10.1002/ehf2.14007. Epub 2022 Jun 6. ESC Heart Fail. 2022. PMID: 35666046 Free PMC article. No abstract available.
Abstract
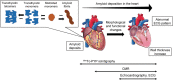
Transthyretin amyloid cardiomyopathy (ATTR-CM) is caused by the cardiac deposition of insoluble amyloid fibrils formed by misfolded transthyretin proteins and is associated with various cardiac symptoms, such as progressive heart failure, conduction disturbance, and arrhythmia. The implementation of 99m technetium (99m Tc)-labelled bone radiotracer scintigraphy for diagnosing ATTR-CM has enabled accurate diagnosis of the disease with high sensitivity and specificity and positioned this diagnostic modality as an integral part of disease diagnostic algorithms. In 2020, 99m Tc-pyrophosphate scintigraphy received exceptional approval for Japanese national health insurance reimbursement as a diagnostic method of ATTR-CM. Nevertheless, the utility of 99m Tc-labelled bone radiotracer scintigraphy and the importance of an early diagnosis of suspected ATTR-CM using this technique have yet to be internalized as common practice by general cardiologists, and guidance on daily clinical scenarios to consider this technique for a diagnosis of suspected ATTR-CM is warranted. In this review, we discuss the utility of 99m Tc-labelled bone radiotracer scintigraphy for the early diagnosis of ATTR-CM based on published literature and the outcomes of an advisory board meeting. This review also discusses clinical scenarios that could support early diagnosis of suspected ATTR-CM as well as common pitfalls, correct implementation, and future perspectives of 99m Tc-labelled bone radiotracer scintigraphy in daily clinical practice. The clinical scenarios to consider 99m Tc-labelled bone radiotracer scintigraphy in daily practice may include, but are not limited to, patients with a family history of the hereditary type of disease; elderly patients (aged ≥60 years) with unexplained cardiac findings (e.g. cardiac hypertrophy associated with abnormalities on an electrocardiogram, heart failure with preserved ejection fraction associated with unexplained left ventricular hypertrophy, and heart failure with reduced ejection fraction associated with atrial fibrillation and left ventricular hypertrophy); and patients with cardiac hypertrophy associated with diastolic dysfunction, right ventricular/interatrial septum/valve thickness, left ventricular sparkling, or apical sparing. Cardiac hypertrophy and persistent elevation in cardiac troponin in elderly patients are also suggestive of ATTR-CM. 99m Tc-labelled bone radiotracer scintigraphy is also recommended in patients with characteristic cardiac magnetic resonance findings (e.g. diffuse subendocardial late gadolinium enhancement patterns, native T1 increase, and increase in extracellular volume) or patients with cardiac hypertrophy and bilateral carpal tunnel syndrome.
Keywords: 99mTechnetium-pyrophosphate scintigraphy; Amyloidosis; Cardiomyopathy; Heart failure; Transthyretin.
© 2021 The Authors. ESC Heart Failure published by John Wiley & Sons Ltd on behalf of European Society of Cardiology.
Conflict of interest statement
Nobuhiro Tahara, Jin Endo, and Atsushi Okada have received consulting fees or honoraria from Pfizer Japan Inc. for the submitted work. Olivier Lairez has received consulting fees or honoraria from Pfizer Japan Inc. for the submitted work and reports financial relationships outside of the submitted work with Alnylam, Amicus Therapeutics, Pfizer, Sanofi‐Genzyme, and Takeda. Mitsuharu Ueda has received consulting fees or honoraria, support for travel to meetings, and administrative support for writing assistance, medicines, or equipment from Pfizer Japan Inc. for the submitted work and reports financial relationships outside of the submitted work with Pfizer Japan Inc. and Alnylam Japan Co., Ltd. Tomonori Ishii, Yoshinobu Kitano, Hahn‐Ey Lee, and Eleonora Russo are full‐time employees of Pfizer Japan Inc. Toru Kubo has received consulting fees or honoraria and remuneration for lecture from Pfizer Japan Inc. for the submitted work.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

