Vangl2-environment interaction causes severe neural tube defects, without abnormal neuroepithelial convergent extension
- PMID: 34842271
- PMCID: PMC8807581
- DOI: 10.1242/dmm.049194
Vangl2-environment interaction causes severe neural tube defects, without abnormal neuroepithelial convergent extension
Abstract
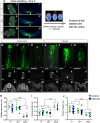
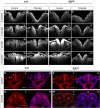
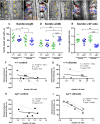
Planar cell polarity (PCP) signalling is vital for initiation of mouse neurulation, with diminished convergent extension (CE) cell movements leading to craniorachischisis, a severe neural tube defect (NTD). Some humans with NTDs also have PCP gene mutations but these are heterozygous, not homozygous as in mice. Other genetic or environmental factors may interact with partial loss of PCP function in human NTDs. We found that reduced sulfation of glycosaminoglycans interacts with heterozygosity for the Lp allele of Vangl2 (a core PCP gene), to cause craniorachischisis in cultured mouse embryos, with rescue by exogenous sulphate. We hypothesized that this glycosaminoglycan-PCP interaction may regulate CE, but, surprisingly, DiO labelling of the embryonic node demonstrates no abnormality of midline axial extension in sulfation-depleted Lp/+ embryos. Positive-control Lp/Lp embryos show severe CE defects. Abnormalities were detected in the size and shape of somites that flank the closing neural tube in sulfation-depleted Lp/+ embryos. We conclude that failure of closure initiation can arise by a mechanism other than faulty neuroepithelial CE, with possible involvement of matrix-mediated somite expansion, adjacent to the closing neural tube.
Keywords: Embryo culture; Glycosaminoglycans; Mouse; Neurulation; Planar cell polarity; Proteoglycans.
© 2022. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures




References
-
- Au, K. S., Hebert, L., Hillman, P., Baker, C., Brown, M. R., Kim, D.-K., Soldano, K., Garrett, M., Ashley-Koch, A., Lee, S.et al. (2021). Human myelomeningocele risk and ultra-rare deleterious variants in genes associated with cilium, WNT-signaling, ECM, cytoskeleton and cell migration. Sci. Rep. 11, 3639. 10.1038/s41598-021-83058-7 - DOI - PMC - PubMed
-
- Bassuk, A. G., Muthuswamy, L. B., Boland, R., Smith, T. L., Hulstrand, A. M., Northrup, H., Hakeman, M., Dierdorff, J. M., Yung, C. K., Long, A.et al. (2013). Copy number variation analysis implicates the cell polarity gene glypican 5 as a human spina bifida candidate gene. Hum. Mol. Genet. 22, 1097-1111. 10.1093/hmg/dds515 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

