The zebrafish embryo as an in vivo model for screening nanoparticle-formulated lipophilic anti-tuberculosis compounds
- PMID: 34842273
- PMCID: PMC8807572
- DOI: 10.1242/dmm.049147
The zebrafish embryo as an in vivo model for screening nanoparticle-formulated lipophilic anti-tuberculosis compounds
Abstract
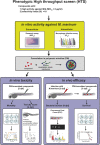
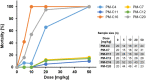
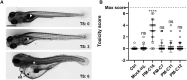
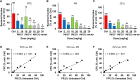
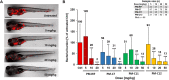
With the increasing emergence of drug-resistant Mycobacterium tuberculosis strains, new and effective antibiotics against tuberculosis (TB) are urgently needed. However, the high frequency of poorly water-soluble compounds among hits in high-throughput drug screening campaigns is a major obstacle in drug discovery. Moreover, in vivo testing using conventional animal TB models, such as mice, is time consuming and costly, and represents a major bottleneck in lead compound discovery and development. Here, we report the use of the zebrafish embryo TB model for evaluating the in vivo toxicity and efficacy of five poorly water-soluble nitronaphthofuran derivatives, which were recently identified as possessing anti-TB activity in vitro. To aid solubilization, compounds were formulated in biocompatible polymeric micelles (PMs). Three of the five PM-formulated nitronaphthofuran derivatives showed low toxicity in vivo, significantly reduced bacterial burden and improved survival in infected zebrafish embryos. We propose the zebrafish embryo TB-model as a quick and sensitive tool for evaluating the in vivo toxicity and efficacy of new anti-TB compounds during early stages of drug development. Thus, this model is well suited for pinpointing promising compounds for further development.
Keywords: In vivo efficacy; In vivo toxicity; Anti-tuberculosis drugs; Nanoparticles; Zebrafish tuberculosis model.
© 2022. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures


References
-
- Andreu, N., Zelmer, A., Sampson, S. L., Ikeh, M., Bancroft, G. J., Schaible, U. E., Wiles, S. and Robertson, B. D. (2013). Rapid in vivo assessment of drug efficacy against Mycobacterium tuberculosis using an improved firefly luciferase. J. Antimicrob. Chemother. 68, 2118-2127. 10.1093/jac/dkt155 - DOI - PMC - PubMed
-
- Balganesh, T. S., Balasubramanian, V. and Kumar, S. A. (2004). Drug discovery for tuberculosis: Bottlenecks and path forward. Curr. Sci. 86, 167-176.
-
- Bandodkar, B., Shandil, R. K., Bhat, J. and Balganesh, T. S. (2020). Two decades of TB drug discovery efforts-what have we learned? Appl. Sci. 10, 5704. 10.3390/app10165704 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

