Glycoengineering of Therapeutic Antibodies with Small Molecule Inhibitors
- PMID: 34842612
- PMCID: PMC8628514
- DOI: 10.3390/antib10040044
Glycoengineering of Therapeutic Antibodies with Small Molecule Inhibitors
Abstract
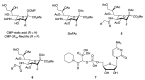
Monoclonal antibodies (mAbs) are one of the cornerstones of modern medicine, across an increasing range of therapeutic areas. All therapeutic mAbs are glycoproteins, i.e., their polypeptide chain is decorated with glycans, oligosaccharides of extraordinary structural diversity. The presence, absence, and composition of these glycans can have a profound effect on the pharmacodynamic and pharmacokinetic profile of individual mAbs. Approaches for the glycoengineering of therapeutic mAbs-the manipulation and optimisation of mAb glycan structures-are therefore of great interest from a technological, therapeutic, and regulatory perspective. In this review, we provide a brief introduction to the effects of glycosylation on the biological and pharmacological functions of the five classes of immunoglobulins (IgG, IgE, IgA, IgM and IgD) that form the backbone of all current clinical and experimental mAbs, including an overview of common mAb expression systems. We review selected examples for the use of small molecule inhibitors of glycan biosynthesis for mAb glycoengineering, we discuss the potential advantages and challenges of this approach, and we outline potential future applications. The main aim of the review is to showcase the expanding chemical toolbox that is becoming available for mAb glycoengineering to the biology and biotechnology community.
Keywords: antibody; chemical tools; glycan; glycoengineering; glycoform; glycosylation; immunoglobulin; inhibitor.
Conflict of interest statement
S.N.K. is founder and shareholder of Epsilogen Ltd. and declares patents on antibody technologies. D.I.R.S. and R.A.G. are employed by Ludger, Ltd., a glycoanalytical company commercializing analytical assays for use in the field of glycomics and the analysis of biopharmaceuticals.
Figures

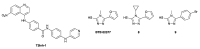
References
Publication types
Grants and funding
- C10355/A15587/NIHR CRUK Experimental Cancer Medicine Centre
- C30122/A15774/CRUK_/Cancer Research UK/United Kingdom
- C30122/A11527/CRUK_/Cancer Research UK/United Kingdom
- IS-BRC-1215-20006/National Institute for Health Research Biomedical Research Centre
- 147; KCL-BCN-Q3/BBC_/Breast Cancer Now/United Kingdom
LinkOut - more resources
Full Text Sources
Miscellaneous

