Electrospun Fibers Derived from Peptide Coupled Amphiphilic Copolymers for Dorsal Root Ganglion (DRG) Outgrowth
- PMID: 34842696
- PMCID: PMC8628770
- DOI: 10.3390/gels7040196
Electrospun Fibers Derived from Peptide Coupled Amphiphilic Copolymers for Dorsal Root Ganglion (DRG) Outgrowth
Abstract
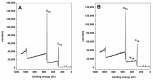
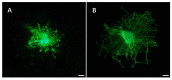
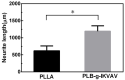
Developing scaffolds with appropriate mechanical/structural features as well as tunable bioactivities are indispensable in the field of tissue engineering. This study focused on one such attempt to electrospin the copolymer of L-lactic acid (L-LA) and functional monomer (3(S)- [(benzyloxycarbony)methyl]-1,4-dioxane-2,5-dione, BMD) with small peptide modifications for the purpose of neural tissue engineering. Scanning Electron Microscopy (SEM) micrographs showed fabricated electrospun copolymer as porous and uniform nanofibrous materials with diameter in the range of 800-1000 nm. In addition, the modified scaffolds displayed a lower contact angle than poly(L-lactide) (PLLA) indicating higher hydrophilicity. To further incorporate the bioactive functions, the nanofibers were chemically coupled with small peptide (isoleucine-lysine-valine-alanine-valine, IKVAV). The incorporation of IKVAV onto the electrospun fiber was confirmed by X-ray photoelectron spectroscopy (XPS) and such incorporation did not affect the surface morphology or fiber diameters. To demonstrate the potential of applying the designed scaffolds for nerve regeneration, dorsal root ganglion (DRG) neurons were cultured on the nanofibers to examine the impact on neurite outgrowth of DRGs. The results indicated that the fabricated nanofibrous matrix with small peptide might be a potential candidate for neural tissue engineering.
Keywords: dorsal root ganglion; electrospun; functional monomer; isoleucine-lysine-valine-alanine-valine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Behtaj S., Karamali F., Masaeli E., Anissimov Y.G., Rybachuk M. Electrospun PGS/PCL, PLLA/PCL, PLGA/PCL and pure PCL scaffolds for retinal progenitor cell cultivation. Biochem. Eng. J. 2021;166:107846. doi: 10.1016/j.bej.2020.107846. - DOI
-
- Barrientos I.J.H., Paladino E., Szabó P., Brozio S., Hall P.J., Oseghale C.I., Passarelli M.K., Moug S.J., Black R.A., Wilson C.G., et al. Electrospun collagen-based nanofibres: A sustainable material for improved antibiotic utilisation in tissue engineering applications. Int. J. Pharm. 2017;531:67–79. doi: 10.1016/j.ijpharm.2017.08.071. - DOI - PubMed
-
- Kang Y.M., Lee S.H., Lee J.Y., Son J.S., Kim B.S., Lee B., Chun H.J., Min B.H., Kim J.H., Kim M.S. A biodegradable, injectable, gel system based on MPEG-b-(PCL-ran-PLLA) diblock copolymers with an adjustable therapeutic window. Biomaterials. 2010;31:2453–2460. doi: 10.1016/j.biomaterials.2009.11.115. - DOI - PubMed
-
- Dorati R., Conti B., Colzani B., Dondi D., Lazzaroni S., Modena T., Genta I. Ivermectin controlled release implants based on poly-D,L-lactide and poly-ε-caprolactone. J. Drug Deliv. Sci. Tec. 2018;46:101–110. doi: 10.1016/j.jddst.2018.04.014. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

