Biomedical Application, Patent Repository, Clinical Trial and Regulatory Updates on Hydrogel: An Extensive Review
- PMID: 34842705
- PMCID: PMC8628667
- DOI: 10.3390/gels7040207
Biomedical Application, Patent Repository, Clinical Trial and Regulatory Updates on Hydrogel: An Extensive Review
Abstract
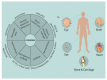
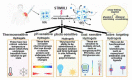
Hydrogels are known for their leading role in biomaterial systems involving pharmaceuticals that fascinate material scientists to work on the wide variety of biomedical applications. The physical and mechanical properties of hydrogels, along with their biodegradability and biocompatibility characteristics, have made them an attractive and flexible tool with various applications such as imaging, diagnosis and treatment. The water-cherishing nature of hydrogels and their capacity to swell-contingent upon a few ecological signals or the simple presence of water-is alluring for drug conveyance applications. Currently, there are several problems relating to drug delivery, to which hydrogel may provide a possible solution. Hence, it is pertinent to collate updates on hydrogels pertaining to biomedical applications. The primary objective of this review article is to garner information regarding classification, properties, methods of preparations, and of the polymers used with particular emphasis on injectable hydrogels. This review also covers the regulatory and other commerce specific information. Further, it enlists several patents and clinical trials of hydrogels with related indications and offers a consolidated resource for all facets associated with the biomedical hydrogels.
Keywords: biomaterial; drug delivery; hydrogel; injectable; patents; regulatory.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Chirani N., Gritsch L., Motta F.L., Fare S. History and Applications of Hydrogels. J. Biomed. Sci. 2015;4 doi: 10.4172/2254-609x.100013. - DOI
-
- Hoare T.R., Kohane D.S. Hydrogels in drug delivery: Progress and challenges. Polymer. 2008;49:1993–2007. doi: 10.1016/j.polymer.2008.01.027. - DOI
-
- Wichterle O., Lím D. Hydrophilic Gels for Biological Use. Nat. Cell Biol. 1960;185:117–118. doi: 10.1038/185117a0. - DOI
Publication types
LinkOut - more resources
Full Text Sources

