In Silico Drug Screening Based Development of Novel Formulations for Onychomycosis Management
- PMID: 34842710
- PMCID: PMC8628710
- DOI: 10.3390/gels7040221
In Silico Drug Screening Based Development of Novel Formulations for Onychomycosis Management
Abstract
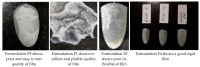
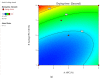
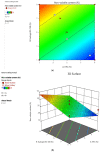
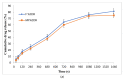
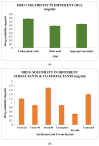
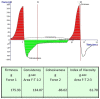
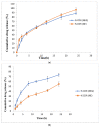
Onychomycosis is a prominent fungal infection that causes discoloration, thickening, and mutilation leading to the separation of the nail from the nail bed. Treatment modalities for onychomycosis may include oral, topical, or combination therapy with antifungals and at times may require chemical or surgical intervention. The burden of side effects of antifungals is enormous, and therefore using molecular docking-based drug selection in context with the target keratin protein would ensure better disease management. Ciclopirox, Amorolfine HCl, Efinaconazole, Tioconazole, and Tavaborole were submitted for assessment, revealing that Amorolfine HCl is the best fit. Consequently, two formulations (Nail lacquer and nanoemulgel) were developed from Amorolfine HCl to validate the in silico screening outcomes. The formulations were further fortified with over-the-counter ingredients vis-a-vis with vitamin E in nail lacquer and undecylenic acid in nanoemulgel for their prominent roles in improving nail health. Both the formulations were systematically designed, optimized, and characterized. Amorolfine HCl containing nanoemulgel (NEG) was developed using undecylenic acid as an oil phase and thioglycolic acid as a penetration enhancer. The quality parameters evaluated were particle size, the zeta potential for nanoemulsion (NE) (78.04 ± 4.724 nm and -0.7mV, respectively), in vitro cumulative drug release (96.74% for NE and 88.54% for NEG), and transungual permeation (about 73.49% for NEG and 54.81% for NE). Nail lacquer was evaluated for the drying time, non-volatile content, and blush test. In vitro cumulative drug release of the developed nail lacquer and comparator marketed formulations were around 81.5% and 75%, respectively. Similarly, the transungual drug permeation was 6.32 μg/cm2 and 5.89 μg/cm2, respectively, in 24 h. The in silico guided preparation of both formulations containing Amorolfine HCl and over the counter ingredients is amenable for therapeutic use against onychomycosis and will be evaluated in the in vivo model.
Keywords: Box–Behnken design; amorolfine HCl; nail lacquer; nanoemulgel; transungual permeation; undecylenic acid; vitamin E.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources

