Metabolic engineering of Vibrio natriegens for anaerobic succinate production
- PMID: 34843164
- PMCID: PMC9151343
- DOI: 10.1111/1751-7915.13983
Metabolic engineering of Vibrio natriegens for anaerobic succinate production
Abstract
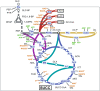
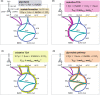
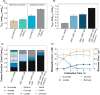
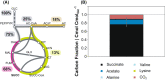
The biotechnological production of succinate bears serious potential to fully replace existing petrochemical approaches in the future. In order to establish an economically viable bioprocess, obtaining high titre, yield and productivity is of central importance. In this study, we present a straightforward engineering approach for anaerobic succinate production with Vibrio natriegens, consisting of essential metabolic engineering and optimization of process conditions. The final producer strain V. natriegens Δlldh Δdldh Δpfl Δald Δdns::pycCg (Succ1) yielded 1.46 mol of succinate per mol of glucose under anaerobic conditions (85% of the theoretical maximum) and revealed a particularly high biomass-specific succinate production rate of 1.33 gSucc gCDW -1 h-1 compared with well-established production systems. By applying carbon and redox balancing, we determined the intracellular flux distribution and show that under the tested conditions the reductive TCA as well as the oxidative TCA/glyoxylate pathway contributed to succinate formation. In a zero-growth bioprocess using minimal medium devoid of complex additives and expensive supplements, we obtained a final titre of 60.4 gSucc l-1 with a maximum productivity of 20.8 gSucc l-1 h-1 and an overall volumetric productivity of 8.6 gSucc l-1 h-1 during the 7 h fermentation. The key performance indicators (titre, yield and productivity) of this first engineering approach in V. natriegens are encouraging and compete with costly tailored microbial production systems.
© 2021 The Authors. Microbial Biotechnology published by Society for Applied Microbiology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that there are no competing interests associated with this work.
Figures
References
-
- Abe, S. , Takayama, K.I. , and Kinoshita, S. (1967) Taxonomical studies on glutamic acid‐producing bacteria. J Gen Appl Microbiol 13: 279–301.
-
- Ahn, J.H. , Jang, Y.‐S. , and Lee, S.Y. (2016) Production of succinic acid by metabolically engineered microorganisms. Curr Opin Biotechnol 42: 54–66. - PubMed
-
- Bozell, J.J. , and Petersen, G.R. (2010) Technology development for the production of biobased products from biorefinery carbohydrates—the US Department of Energy’s “top 10” revisited. Green Chem 12: 539–555.
-
- Buchholz, J. , Graf, M. , Blombach, B. , and Takors, R. (2014) Improving the carbon balance of fermentations by total carbon analyses. Biochem Eng J 90: 162–169.
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

