Poziotinib in Non-Small-Cell Lung Cancer Harboring HER2 Exon 20 Insertion Mutations After Prior Therapies: ZENITH20-2 Trial
- PMID: 34843401
- PMCID: PMC8887939
- DOI: 10.1200/JCO.21.01323
Poziotinib in Non-Small-Cell Lung Cancer Harboring HER2 Exon 20 Insertion Mutations After Prior Therapies: ZENITH20-2 Trial
Abstract
Purpose: Insertion mutations in Erb-b2 receptor tyrosine kinase 2 gene (ERBB2 or HER2) exon 20 occur in 2%-5% of non-small-cell lung cancers (NSCLCs) and function as an oncogenic driver. Poziotinib, a tyrosine kinase inhibitor, was evaluated in previously treated patients with NSCLC with HER2 exon 20 insertions.
Methods: ZENITH20, a multicenter, multicohort, open-label phase II study, evaluated poziotinib in patients with advanced or metastatic NSCLC. In cohort 2, patients received poziotinib (16 mg) once daily. The primary end point was objective response rate evaluated by independent review committee (RECIST v1.1); secondary outcome measures were disease control rate, duration of response, progression-free survival, and safety and tolerability. Quality of life was assessed.
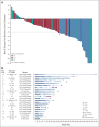
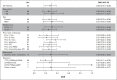
Results: Between October 2017 and March 2021, 90 patients with a median of two prior lines of therapy (range, 1-6) were treated. With a median follow-up of 9.0 months, objective response rate was 27.8% (95% CI, 18.9 to 38.2); 25 of 90 patients achieved a partial response. Disease control rate was 70.0% (95% CI, 59.4 to 79.2). Most patients (74%) had tumor reduction (median reduction 22%). Median progression-free survival was 5.5 months (95% CI, 3.9 to 5.8); median duration of response was 5.1 months (95% CI, 4.2 to 5.5). Clinical benefit was seen regardless of lines and types of prior therapy, presence of central nervous system metastasis, and types of HER2 mutations. Grade 3 or higher treatment-related adverse events included rash (48.9%), diarrhea (25.6%), and stomatitis (24.4%). Most patients had poziotinib dose reductions (76.7%), with median relative dose intensity of 71.5%. Permanent treatment discontinuation because of treatment-related adverse events occurred in 13.3% of patients.
Conclusion: Poziotinib demonstrates antitumor activity in previously treated patients with HER2 exon 20 insertion NSCLC.
Trial registration: ClinicalTrials.gov NCT03318939.
Conflict of interest statement
Figures
Comment in
-
Human Epidermal Growth Factor Receptor 2-Mutant Non-Small-Cell Lung Cancer: Continued Progress But Challenges Remain.J Clin Oncol. 2022 Mar 1;40(7):693-697. doi: 10.1200/JCO.21.02550. Epub 2022 Jan 24. J Clin Oncol. 2022. PMID: 35072488 No abstract available.
References
-
- Lee JW, Soung YH, Seo SH, et al. : Somatic mutations of ERBB2 kinase domain in gastric, colorectal, and breast carcinomas. Clin Cancer Res 12:57-61, 2006 - PubMed
-
- Mazières J, Peters S, Lepage B, et al. : Lung cancer that harbors an HER2 mutation: Epidemiologic characteristics and therapeutic perspectives. J Clin Oncol 31:1997-2003, 2013 - PubMed
-
- Mazières J, Barlesi F, Filleron T, et al. : Lung cancer patients with HER2 mutations treated with chemotherapy and HER2-targeted drugs: Results from the European EUHER2 cohort. Ann Oncol 27:281-286, 2016 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

