Evaluation of a novel, rapid antigen detection test for the diagnosis of SARS-CoV-2
- PMID: 34843505
- PMCID: PMC8629250
- DOI: 10.1371/journal.pone.0259527
Evaluation of a novel, rapid antigen detection test for the diagnosis of SARS-CoV-2
Abstract
Background: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causing coronavirus disease 2019 (COVID-19) is currently finally determined in laboratory settings by real-time reverse-transcription polymerase-chain-reaction (rt-PCR). However, simple testing with immediately available results are crucial to gain control over COVID-19. The aim was to evaluate such a point-of-care antigen rapid test (AG-rt) device in its performance compared to laboratory-based rt-PCR testing in COVID-19 suspected, symptomatic patients.
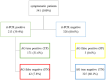
Methods: For this prospective study, two specimens each of 541 symptomatic female (54.7%) and male (45.3%) patients aged between 18 and 95 years tested at five emergency departments (ED, n = 296) and four primary healthcare centres (PHC, n = 245), were compared, using AG-rt (positive/negative/invalid) and rt-PCR (positive/negative and cycle threshold, Ct) to diagnose SARS-CoV-2. Diagnostic accuracy, sensitivity, specificity, positive predictive values (PPV), negative predictive value (NPV), and likelihood ratios (LR+/-) of the AG-rt were assessed.
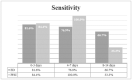
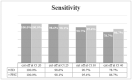
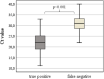
Results: Differences between ED and PHC were detected regarding gender, age, symptoms, disease prevalence, and diagnostic performance. Overall, 174 (32.2%) were tested positive on AG-rt and 213 (39.4%) on rt-PCR. AG correctly classified 91.7% of all rt-PCR positive cases with a sensitivity of 80.3%, specificity of 99.1%, PPV of 98.3, NPV of 88.6%, LR(+) of 87.8, and LR(-) of 0.20. The highest sensitivities and specificities of AG-rt were detected in PHC (sensitivity: 84.4%, specificity: 100.0%), when using Ct of 30 as cut-off (sensitivity: 92.5%, specificity: 97.8%), and when symptom onset was within the first three days (sensitivity: 82.9%, specificity: 99.6%).
Conclusions: The highest sensitivity was detected with a high viral load. Our findings suggest that AG-rt are comparable to rt-PCR to diagnose SARS-CoV-2 in COVID-19 suspected symptomatic patients presenting both at emergency departments and primary health care centres.
Conflict of interest statement
The authors have read the journal’s policy and have the following competing interests: Roche Diagnostics provided support for this study in the form of funds sent to the scientific association Science Center Donaustadt, which were used to cover the costs of the test materials and statistician. There are no patents, products in development or marketed products associated with this research to declare. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
References
-
- CDC. Centers for Disease Control and Prevention. ARS-CoV-2 Testing Strategy: Considerations for Non-Healthcare Workplaces. 21 October 2020.
-
- ECDPC. European Centre for Disease Prevention and Control. COVID-19 testing strategies and objectives. 15 September 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

