Pan-Asian adaptation of the EHNS-ESMO-ESTRO Clinical Practice Guidelines for the diagnosis, treatment and follow-up of patients with squamous cell carcinoma of the head and neck
- PMID: 34844180
- PMCID: PMC8710460
- DOI: 10.1016/j.esmoop.2021.100309
Pan-Asian adaptation of the EHNS-ESMO-ESTRO Clinical Practice Guidelines for the diagnosis, treatment and follow-up of patients with squamous cell carcinoma of the head and neck
Abstract
The most recent version of the European Society for Medical Oncology (ESMO) Clinical Practice Guidelines for the diagnosis, treatment and follow-up of squamous cell carcinoma (SCC) of the oral cavity, larynx, oropharynx and hypopharynx was published in 2020. It was therefore decided by both the ESMO and the Korean Society of Medical Oncology (KSMO) to convene a special, virtual guidelines meeting in July 2021 to adapt the ESMO 2020 guidelines to consider the potential ethnic differences associated with the treatment of SCCs of the head and neck (SCCHN) in Asian patients. These guidelines represent the consensus opinions reached by experts in the treatment of patients with SCCHN (excluding nasopharyngeal carcinomas) representing the oncological societies of Korea (KSMO), China (CSCO), India (ISMPO), Japan (JSMO), Malaysia (MOS), Singapore (SSO) and Taiwan (TOS). The voting was based on scientific evidence and was independent of the current treatment practices and drug access restrictions in the different Asian countries. The latter was discussed when appropriate. This manuscript provides a series of expert recommendations (Clinical Practice Guidelines) which can be used to provide guidance to health care providers and clinicians for the optimisation of the diagnosis, treatment and management of patients with SCC of the oral cavity, larynx, oropharynx and hypopharynx across Asia.
Keywords: ESMO; Pan-Asian; guidelines; head and neck; squamous cell carcinoma; treatment.
Copyright © 2021 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Disclosure BK declares grants or contracts from Merck Sharp & Dohme (MSD) Oncology, Ono Pharmaceutical and AstraZeneca, consulting fees from AstraZeneca, MSD Oncology, ABL Bio, Genexine, Cellid, Handok, Celid, Trial Informatics and CBS Bio, payment or honoraria from MSD Oncology and Merck. J-PM declares consulting fees/honoraria from Pfizer, Roche, AstraZeneca, Bayer, Innate, Merck Serono, Boehringer, Bristol Myers Squibb (BMS), Novartis, Janssen, Incyte, Cue Biopharma, ALX Oncology, iTEOS, TheRNA and NEKTAR, support for attending meetings and/or travel from Amgen, Pfizer and MSD and participation at a Safety or Advisory Board for PsiOxus. LL declares institutional grants or contracts from AstraZeneca, BMS, Boehringer Ingelheim, Celgene International, Eisai, Exelixis Inc, Debiopharm International SA, Hoffman-La Roche Ltd., IRX Therapeutics Inc., Medpace Inc., Merck-Serono, MSD, Novartis, Pfizer, Roche Spa and Buran and receipt of honoraria or fees (for public speaking/teaching in medical meetings and/or for providing expert opinion in Advisory Boards) for AstraZeneca, Bayer, BMS, Eisai, MSD, Merck-Serono, Boehringer Ingelheim, Hoffman La Roche Ltd., Novartis, Roche, Debiopharm International SA, Sobi, Incyte Biosciences Italy SRL, Doxa Pharma, Amgen, Nanobiotics and GlaxoSmithKline (GSK). CB declares payment or honoraria from Merck KGaA, BMS and Roche. MT declares consulting fees from Ono Pharmaceuticals, MSD, BMS and Merck Biopharma, and honoraria from Eisai, Ono Pharmaceuticals, BMS and Merck Biopharma. MA declares consulting fees from MSD, AstraZeneca, Eli Lilly, DKSH, Eisai, Roche, Novartis and Merck, payment or honoraria from MSD, AstraZeneca, Eli Lilly, DKSH, Eisai, Roche, Novartis and Merck, support for attending meetings and/or travel from MSD, Roche, Elekta/Abex and AstraZeneca, and is the president of the Malaysian Oncological Society. MKA declares honoraria for presentations from Pfizer and Boehringer Ingelheim, sponsorship for meetings from AstraZeneca, Boehringer Ingelheim and DKSH. QSNg declares support for attending meetings and or travel from BMS, Boehringer Ingelheim, MSD and Astellas, and participation in Safety or Advisory Boards for MSD and Boehringer Ingelheim. WIWZ declares honoraria for lectures from Amgen Malaysia, DKSH Malaysia, Eisai Malaysia, Eli Lilly Malaysia, Ipsen Malaysia, MSD Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, Merck Serono Malaysia, Pfizer Malaysia and Roche Malaysia, travel grants from Amgen Malaysia, Celgene Malaysia, Eisai Malaysia, Eli Lilly Malaysia, MSD Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and Roche Malaysia, participation on an Advisory Board for Celgene Malaysia, Roche Malaysia, Eli Lilly Malaysia, Eisai Malaysia and MSD Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and is a member of ASCO AP Regional Council and Greater Petaling Cancer City Challenge UICC. NK declares institutional grants or contracts from Ono Pharmaceutical, BMS, AstraZeneca, Pfizer, Chugai Pharmaceutical, Rakuten Medical, Bayer and Adlai Nortye, payment or honoraria from Ono Pharmaceutical, BMS, Merck Biopharma, MSD, Eisai and Bayer, participation on a Data Safety Monitoring Board or Advisory Board for Bayer and Adlai Nortye. GC declares institutional grants from Merck, consulting fees from BMS, Pfizer, MSD, AstraZeneca, Daichii Sankyo, Lilly, Novartis and Seattle Genetics, payment or honoraria from AstraZeneca, Roche and Daichii Sankyo. SP declares fees for consultancy/advisory roles from AbbVie, Amgen, AstraZeneca, Bayer, Beigene, Biocartis, Boehringer Ingelheim, BMS, Clovis, Daiichi Sankyo, Debiopharm, ecancer, Eli Lilly, Elsevier, Foundation Medicine, Illumina, Imedex, Incyte, Janssen, Medscape, MSD, Merck Serono, Merrimack, Novartis, PharmaMar, Phosplatin Therapeutics, PER, Pfizer, PRIME, Regeneron, Roche/Genentech, RTP, Sanofi, Seattle Genetics, Takeda, speaker roles for AstraZeneca, Boehringer Ingelheim, BMS, ecancer, Eli Lilly, Illumina, Imedex, Medscape, MSD, Novartis, PER, Pfizer, Prime, Roche/Genentech, RTP, Sanofi, Takeda and the receipt of grants/research support: (Sub) investigator in trials (institutional financial support for trials) sponsored by Amgen, AstraZeneca, Biodesix, Boehringer Ingelheim, BMS, Clovis, GSK, Illumina, Lilly, MSD, Merck Serono, Mirati, Novartis, and Pfizer, Phosplatin Therapeutics, Roche/Genentech. TWK declares institutional grants or contracts from Roche, and sanofi-aventis. TY declares institutional grants or contracts from Taiho Pharmaceutical, Sumitomo Dainippon, Ono Pharmaceutical, Chugai Pharmaceutical, Amgen, Parexel International, MSD, Daiichi-Sankyo and Sanofi. All other authors have declared no conflicts of interest.
Figures
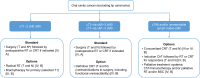
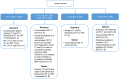
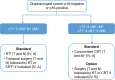
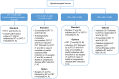
References
-
- Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. - PubMed
-
- Zhang L.-W., Li J., Cong X., et al. Incidence and mortality trends in oral and oropharyngeal cancer in China, 2005-2013. Cancer Epidemiol. 2018;57:120–126. - PubMed
-
- Hwang T.Z., Hsiao J.R., Tsai C.R., Chang J.S. Incidence trends of human papillomavirus-related head and neck cancer in Taiwan, 1995-2009. Int J Cancer. 2015;137:395–408. - PubMed
-
- Vital Statistics Japan. In: Ministry of Health Law (ed) Edition 2018. https://ganjoho.jp/reg stat/statistsics/index.html Available at.

