Continuous glucose monitoring systems for monitoring cystic fibrosis-related diabetes
- PMID: 34844283
- PMCID: PMC8629645
- DOI: 10.1002/14651858.CD013755.pub2
Continuous glucose monitoring systems for monitoring cystic fibrosis-related diabetes
Abstract
Background: Cystic fibrosis (CF) is one of the most common life-shortening autosomal-recessive genetic conditions with around 100,000 people affected globally. CF mainly affects the respiratory system, but cystic fibrosis-related diabetes (CFRD) is a common extrapulmonary co-morbidity and causes excess morbidity and mortality in this population. Continuous glucose monitoring systems (CGMS) are a relatively new technology and, as yet, the impact of these on the monitoring and subsequent management of CFRD remains undetermined.
Objectives: To establish the impact of insulin therapy guided by continuous glucose monitoring compared to insulin therapy guided by other forms of glucose data collection on the lives of people with CFRD.
Search methods: We searched the Cochrane Cystic Fibrosis Trials Register, compiled from electronic database searches and handsearching of journals and conference abstract books. Date of latest search: 23 September 2021. We also searched the reference lists of relevant articles and reviews and online trials registries. Date of last search: 23 September 2021.
Selection criteria: Randomised controlled studies comparing insulin regimens led by data from CGMS (including real-time or retrospective data, or both) with insulin regimens guided by abnormal blood glucose measurements collected through other means of glycaemic data collection in people with CFRD. Studies with a cross-over design, even with a washout period between intervention arms, are not eligible for inclusion due to the potential long-term impact of each of the interventions and the potential to compromise the outcomes of the second intervention.
Data collection and analysis: No studies were included in the review, meaning that no data were available to be collected for analysis.
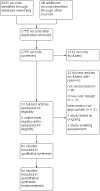
Main results: Review authors screened 14 studies at the full-text stage against the review's inclusion criteria. Consequently, seven were excluded due to the study type being ineligible (not randomised), two studies were excluded due to their cross-over design, and two studies was excluded since the intervention used was not eligible and one was a literature review. One study in participants hospitalised for a pulmonary exacerbation is ongoing. Investigators are comparing insulin dosing via insulin pump with blood sugar monitoring by a CGMS to conventional diabetes management with daily insulin injections (or on an insulin pump if already on an insulin pump in the outpatient setting) and capillary blood glucose monitoring. The participants in the control arm will wear a blinded continuous glucose monitoring system for outcome assessment. In addition to this, one further study is still awaiting classification, and will be screened to determine whether it is eligible for inclusion, or is to be excluded, in an update of this review.
Authors' conclusions: No studies were included in the review, indicating that there is currently insufficient evidence to determine the impact of insulin therapy guided by CGMS compared to insulin therapy guided by other forms of glucose data collection on the lives of people with CFRD, nor on potential adverse effects of continuous glucose monitoring in this context. Randomised controlled studies are needed to generate evidence on the efficacy and safety of continuous glucose monitoring in people with CFRD. There is one relevant ongoing study that may be eligible for inclusion in a future update of this Cochrane Review, and whose results may help answer the review question.
Trial registration: ClinicalTrials.gov NCT03939065.
Copyright © 2021 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
All authors: none known.
Update of
References
References to studies excluded from this review
EUCTR2004‐005019‐28‐GB {published data only}
-
- EUCTR2004-005019-28-GB. Diabetes therapy to improve body mass index pulmoanry function in cystic fibrosis subjects with abnormal blood glucose. www.clinicaltrialsregister.eu/ctr-search/search?query=EUCTR2004-005019-2... (start date 09 May 2005).
Guilbert 2018 {published data only}
-
- Guilbert L, Arregui-Fresneda I, Daniels T, Holt RI. A service evaluation of the use of flash glucose monitoring in people with cystic fibrosis-related diabetes (CFRD). Diabetic Medicine 2018;35:74.
Hagan 2020 {published data only}
-
- Hagan OM, McDermott AJ, Stackhouse AC, Spillman NL. The impact of FreeStyle Libre flash glucose monitoring system on HbA1c in patients with cystic fibrosis-related diabetes. Journal of Cystic Fibrosis 2020;19(Suppl 2):126. [ABSTRACT NO.: P250] [DOI: 10.1016/S1569-1993(20)30582-8] - DOI
Jackson 2017 {published data only}
-
- Jackson VM, Dyce P, Walshaw MJ. Exploring the utility of continuous glucose monitoring (CGMS) for cystic fibrosis related diabetes (CFRD). Journal of Cystic Fibrosis 2017;16 Suppl 1:S137. [ABSTRACT NO.: 294]
Jones 2016 {published data only}
NCT04533646 {published data only}
-
- NCT04533646. Comparison of meal-time dosing of insulin in cystic fibrosis related diabetes. clinicaltrials.gov/ct2/show/NCT04533646 (date first posted 31 August 2020).
O'Riordan 2009 {published data only}
Rahman 2020 {published data only}
-
- Rahman S, Mcdermott J, Adler A, Barker H, Johnson C, Grogono D, et al. Impact of flash glucose monitoring system in cystic fibrosis-related diabetes. European Respiratory Journal 2020;56:2774.
Sherwood 2020 {published data only}
Shimmin 2020 {published data only}
-
- Shimmin D, Mansfield M, Taylor K, Pearson L, Lim YW, Etherington C, et al. Early experience of flash glucose monitoring using the Freestyle Libre System in adults with Cystic Fibrosis-Related Diabetes (CFRD) attending a large regional UK centre. Journal of Cystic Fibrosis 2020;19 Suppl 2:S26. [ABSTRACT NO.: WS15.4]
Stackhouse 2017 {published data only}
-
- Stackhouse CA, Floto R, Haworth CS, Shafi NT, Buczak V, Johnson CM, et al. The impact of continuous glucose monitoring on HbA1c in patients with cystic fibrosis related diabetes. Journal of Cystic Fibrosis 2017;16 Suppl 1:148. [ABSTRACT NO.: 336]
References to studies awaiting assessment
NCT03258853 {published data only}
-
- NCT03258853. Feasibility of outpatient closed loop control with the iLet bionic pancreas in cystic fibrosis related diabetes. clinicaltrials.gov/ct2/show/NCT03258853 (date first posted 23 August 2017).
References to ongoing studies
NCT03939065 {unpublished data only}
-
- NCT03939065. Sensor Augmented Pump (SAP) Therapy for Inpatient CFRD Management. clinicaltrials.gov/ct2/show/NCT03939065 (first posted 06 May 2019).
Additional references
ADA 2020
-
- American Diabetes Association. 7. Diabetes Technology: Standards of Medical Care in Diabetes 2020. Diabetes Care 2020;43:S77-88. - PubMed
Ang 2020
Atkins 2004
Baker 2018
-
- Baker EH, Baines DL. Airway glucose homeostasis: a new target in the prevention and treatment of pulmonary infection. Chest 2018;153(2):507-14. - PubMed
Ballmann 2014
Battelino 2019
Bolinder 2016
Brennan 2007
-
- Brennan AL, Gyi Khin M, Wood DM, Johnson J, Holliman R, Baines DL, et al. Airway glucose concentrations and effect on growth of respiratory pathogens in cystic fibrosis. Journal of Cystic Fibrosis 2007;6(2):101-9. - PubMed
Bridges 2018
-
- Bridges N, Rowe R, Holt RIG. Unique challenges of cystic fibrosis-related diabetes. Diabetic Medicine 2018;9:1181. - PubMed
Chan 2019
-
- Chan CL, Ode KL, Granados A, Moheet A, Moran A, Hameed S. Continuous glucose monitoring in cystic fibrosis: a practical guide. Journal of Cystic Fibrosis 2019;18(Suppl 2):S25-31. - PubMed
Cohen 1988
-
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd edition. Hillsdale, New Jersey, USA: L. Erlbaum Associates, 1988. [ISBN: 9780805802832 0805802835]
Cystic Fibrosis Foundation 2019
-
- Cystic Fibrosis Foundation. Cystic Fibrosis Foundation Patient Registry 2018. www.cff.org/Research/Researcher-Resources/Patient-Registry/2018-Patient-... (accessed 05 October 2020).
Damiano 2014
Davies 2020
Deeks 2021
-
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Dobson 2004
-
- Dobson L, Sheldon CD, Hattersley AT. Conventional measures underestimate glycaemia in cystic fibrosis patients. Diabetic Medicine 2004;21(7):691-6. - PubMed
Dodge 2007
-
- Dodge JA, Lewis PA, Stanton M, Wilsher J. Cystic fibrosis mortality and survival in the UK: 1947-2003. European Respiratory Journal 2007;29(3):522-6. - PubMed
Donaghue 2019
-
- Donaghue K, Robinson P. Cystic fibrosis-related diabetes mellitus. www.uptodate.com/contents/cystic-fibrosis-related-diabetes-mellitus#! (accessed 05 October 2020).
Farrell 2018
-
- Farrell P, Férec C, Macek M, Frischer T, Renner S, Riss K, et al. Estimating the age of p.(Phe508del) with family studies of geographically distinct European populations and the early spread of cystic fibrosis. European Journal of Human Genetics 2018;26(12):1832-9. [DOI: 10.1038/s41431-018-0234-z] - DOI - PMC - PubMed
Finkelstein 1988
-
- Finkelstein SM, Wielinski CL, Elliott GR, Warwick WJ, Barbosa J, Wu SC, et al. Diabetes mellitus associated with cystic fibrosis. Journal of Pediatrics 1988;112(3):373-7. - PubMed
Frost 2018
-
- Frost F, Dyce P, Nazareth D, Malone V, Walshaw MJ. Continuous glucose monitoring guided insulin therapy is associated with improved clinical outcomes in cystic fibrosis-related diabetes. Journal of Cystic Fibrosis 2018;17(6):798-803. - PubMed
Gaines 2021
Hardin 1999
-
- Hardin DS, Moran A. Diabetes mellitus in cystic fibrosis. Endocrinology and Metabolism Clinics of North America 1999;28(4):787-800, ix. - PubMed
Hart 2018
Higgins 2017
-
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Higgins 2021
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
ISPAD 2018
-
- Moran A, Pillay K, Becker D, Granados A, Hameed S, Acerini CL. ISPAD clinical practice consensus guidelines 2018: management of cystic fibrosis-related diabetes in children and adolescents. Pediatric Diabetes 2018;19 Suppl 27:64-74. - PubMed
Jefferies 2005
-
- Jefferies C, Solomon M, Perlman K, Sweezey N, Daneman D. Continuous glucose monitoring in adolescents with cystic fibrosis. Journal of Pediatrics 2005;147(3):396-8. - PubMed
Jerusalem 1995
-
- Jerusalem M, Schwarzer R. General Self-Efficacy Scale (Revised English Version). userpage.fu-berlin.de/~health/selfscal.htm (accessed 07 May 2020).
Koch 2001
-
- Koch C, Rainisio M, Madessani U, Harms HK, Hodson ME, Mastella G, et al. Presence of cystic fibrosis-related diabetes mellitus is tightly linked to poor lung function in patients with cystic fibrosis: data from the European Epidemiologic Registry of Cystic Fibrosis. Pediatric Pulmonology 2001;32(5):343-50. - PubMed
Langendam 2012
Lanng 1995
Limoli 2016
-
- Limoli DH, Yang J, Khansaheb MK, Helfman B, Peng L, Stecenko AA, et al. Staphylococcus aureus and Pseudomonas aeruginosa co-infection is associated with cystic fibrosis-related diabetes and poor clinical outcomes. European Journal of Clinical Microbiology & Infectious Diseases 2016;35(6):947-53. [DOI: 10.1007/s10096-016-2621-0] - DOI - PubMed
Maahs 2016
Marshall 2005
-
- Marshall BC, Butler SM, Stoddard M, Moran AM, Liou TG, Morgan WJ. Epidemiology of cystic fibrosis-related diabetes. Journal of Pediatrics 2005;146(5):681-7. - PubMed
Mehfooz 2019
-
- Mehfooz A, Zeb M, Morey-Vargas O. SUN-LB026 Do CFTR Modulators treat cystic fibrosis-related diabetes? Journal of the Endocrine Society 2019;3(1):1. [DOI: 10.1210/js.2019-sun-lb026]
Mohan 2008
-
- Mohan K, Israel K, Miller H, Grainger R, Ledson M, Walshaw M. Long-term effect of insulin treatment in cystic fibrosis-related diabetes. Respiration 2008;76(2):181-6. - PubMed
Moran 1994
-
- Moran A, Pyzdrowski KL, Weinreb J, Kahn BB, Smith SA, Adams KS, et al. Insulin sensitivity in cystic fibrosis. Diabetes 1994;43(8):1020-6. - PubMed
Moran 2009
Moran 2010
-
- Moran A, Brunzell C, Cohen RC, Katz M, Marshall BC, Onady G, et al. Clinical care guidelines for cystic fibrosis–related diabetes: a position statement of the American Diabetes Association and a clinical practice guideline of the Cystic Fibrosis Foundation, endorsed by the Pediatric Endocrine Society. Diabetes Care 2010;33(12):2697-708. [DOI: 10.2337/dc10-1768] - DOI - PMC - PubMed
Moreau 2008
-
- Moreau F, Weiller MA, Rosner V, Weiss L, Hasselmann M, Pinget M, et al. Continuous glucose monitoring in cystic fibrosis patients according to the glucose tolerance. Hormone and Metabolic Research 2008;40(7):502-6. - PubMed
New Zealand Ministry of Health 2014
-
- New Zealand Ministry of Health. Quality standards for diabetes care toolkit 2014. www.health.govt.nz/publication/quality-standards-diabetes-care-toolkit-2014 (accessed 05 October 2020).
NICE 2017
-
- National Institute for Health and Care Excellence (NICE). Cystic fibrosis: diagnosis and management. www.ncbi.nlm.nih.gov/books/NBK464183/ (accessed 17 December 2019). - PubMed
Niu 2016
-
- Niu B, Aviram A, Valent A, Caughey AB. Cost-effectiveness of continuous glucose monitoring vs self-monitoring of blood glucose in women with pre-gestational diabetes. American Journal of Obstetrics and Gynecology 2016;214(1 Suppl):S389. [ABSTRACT NO.: 743]
Onady 2016
Page 2021
-
- Page MJ, Higgins JP, Sterne JA. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Pitocco 2012
Pleus 2019
Ratjen 2003
-
- Ratjen F, Döring G. Cystic fibrosis. Lancet 2003;361(9358):681-9. - PubMed
Regnault 2012
-
- Regnault A, Balp M, Kulich K, Viala-Danten M. Validation of the treatment satisfaction questionnaire for medication in patients with cystic fibrosis. Journal of Cystic Fibrosis 2012;11(6):494-501. - PubMed
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rodman 1986
-
- Rodman HM, Doershuk CF, Roland JM. The interaction of 2 diseases: diabetes mellitus and cystic fibrosis. Medicine 1986;65(6):389-97. - PubMed
Rosenecker 1995
-
- Rosenecker J, Eichler I, Kühn L, Harms HK, Hardt H. Genetic determination of diabetes mellitus in patients with cystic fibrosis. Multicenter Cystic Fibrosis Study Group. Journal of Pediatrics 1995;127(3):441-3. - PubMed
Sawicki 2013
Stutchfield 1988
Tanenberg 2004
-
- Tanenberg R, Bode B, Lane W, Levetan C, Mestman J, Harmel AP, et al. Use of the continuous glucose monitoring system to guide therapy in patients with insulin-treated diabetes: a randomized controlled trial. Mayo Clinic Proceedings 2004;79(12):1521-6. - PubMed
UK Cystic Fibrosis Trust 2020
-
- UK Cystic Fibrosis Trust. Cystic Fibrosis FAQs. www.cysticfibrosis.org.uk/what-is-cystic-fibrosis/faqs# (accessed 05 October 2020).
Volkova 2020
Waugh 2012
Widger 2013
-
- Widger J, Ranganathan S, Robinson PJ. Progression of structural lung disease on CT scans in children with cystic fibrosis related diabetes. Journal of Cystic Fibrosis 2013;12(3):216-21. - PubMed
Yohannes 2011
References to other published versions of this review
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical