Interventions for the management of abdominal pain in Crohn's disease and inflammatory bowel disease
- PMID: 34844288
- PMCID: PMC8629648
- DOI: 10.1002/14651858.CD013531.pub2
Interventions for the management of abdominal pain in Crohn's disease and inflammatory bowel disease
Abstract
Background: Crohn's disease is a remitting and relapsing disorder that can affect the whole gastrointestinal tract. Active disease symptoms include abdominal pain, fatigue, weight loss, and diarrhoea. There is no known cure; however, the disease can be managed, and therefore places a huge financial burden on healthcare systems. Abdominal pain is a common and debilitating symptom of Crohn's and other inflammatory bowel diseases (IBDs), and is multifaceted. Abdominal pain in Crohn's disease could be a symptom of disease relapse or related to medication adverse effects, surgical complications and strictures or adhesions secondary to IBD. In the absence of these factors, around 20 to 50% of people with Crohn's in remission still experience pain.
Objectives: To assess the efficacy and safety of interventions for managing abdominal pain in people with Crohn's disease and IBD (where data on ulcerative colitis and Crohn's disease could not be separated).
Search methods: We searched CENTRAL, MEDLINE, three other databases, and clinical trials registries on 29 April 2021. We also searched the references of trials and systematic reviews for any additional trials.
Selection criteria: All published, unpublished, and ongoing randomised trials that compared interventions for the management of abdominal pain in the setting of Crohn's disease and IBD, with other active interventions or standard therapy, placebo, or no therapy were included. We excluded studies that did not report on any abdominal pain outcomes.
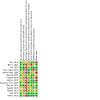
Data collection and analysis: Five review authors independently conducted data extraction and 'Risk of bias' assessment of the included studies. We analysed data using Review Manager 5. We expressed dichotomous and continuous outcomes as risk ratios and mean differences with 95% confidence intervals. We assessed the certainty of the evidence using GRADE methodology.
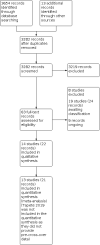
Main results: We included 14 studies (743 randomised participants). Five studies evaluated participants with Crohn's disease; seven studies evaluated participants with IBD where the data on ulcerative colitis and Crohn's disease could not be separated; and two studies provided separate results for Crohn's disease participants. Studies considered a range of disease activity states. Two studies provided intervention success definitions, whilst the remaining studies measured pain as a continuous outcome on a rating scale. All studies except one measured pain intensity, whilst three studies measured pain frequency. Withdrawals due to adverse events were directly or indirectly reported in 10 studies. No conclusions could be drawn about the efficacy of the majority of the interventions on pain intensity, pain frequency, and treatment success, except for the comparison of transcranial direct current stimulation to sham stimulation. The certainty of the evidence was very low in all but one comparison because of imprecision due to sparse data and risk of bias assessed as unclear or high risk. Two studies compared a low FODMAP diet (n=37) to a sham diet (n=45) in IBD patients. The evidence on pain intensity was of very low certainty (MD -12.00, 95% CI -114.55 to 90.55). One study reported pain intensity separately for CD participants in the low FODMAP group [n=14, mean(SD)=24 (82.3)] and the sham group [n=12, mean(SD)=32 (69.3)]. The same study also reported pain frequency for IBD participants in the low FODMAP group [n=27, mean(SD)=36 (26)] and sham group [n=25, mean(SD)=38(25)] and CD participants in the low FODMAP group [n=14, mean(SD)=36 (138.4)] and sham group [n=12, mean(SD)=48 (128.2)]. Treatment success was not reported. One study compared a low FODMAP diet (n=25) to high FODMAP/normal diet (n=25) in IBD patients. The data reported on pain intensity was unclear. Treatment success and pain frequency were not reported. One study compared medicine-separated moxibustion combined with acupuncture (n=51) versus wheat bran-separated moxibustion combined with shallow acupuncture (n=51) in CD patients. The data reported on pain intensity and frequency were unclear. Treatment success was not reported. One study compared mindfulness with CBT (n=33) versus no treatment (n=33) in IBD patients. The evidence is very uncertain about the effect of this treatment on pain intensity and frequency (MD -37.00, 95% CI -87.29 to 13.29). Treatment success was not reported. One study compared soft non-manipulative osteopathic treatment (n=16) with no treatment besides doctor advice (n=14) in CD patients. The evidence is very uncertain about the effect of this treatment on pain intensity (MD 0.01, 95% CI -1.81 to 1.83). Treatment success and pain frequency were not reported. One study compared stress management (n=15) to self-directed stress management(n=15) and to standard treatment (n=15) in CD patients. The evidence is very uncertain about the effect of these treatments on pain intensity (MD -30.50, 95% CI -58.45 to -2.55 and MD -34.30, 95% CI -61.99 to -6.61). Treatment success and pain frequency were not reported. One study compared enteric-release glyceryl trinitrate (n=34) with placebo (n=36) in CD patients. The data reported on pain intensity was unclear. Treatment success and pain frequency were not reported. One study compared 100 mg olorinab three times per day (n=8) with 25 mg olorinab three times per day (n=6) in CD patients. Pain intensity was measured as a 30% reduction in weekly average abdominal pain intensity score for the 100mg group (n=5) and the 25mg group (n=6). The evidence is very uncertain about the effect of this treatment on pain intensity (RR 0.66, 95% CI 0.38 to 1.15). Treatment success and pain frequency were not reported. One study compared relaxation training (n=28) to a waitlist (n=28) in IBD patients. The evidence is very uncertain about the effect of this treatment on pain intensity (MD -0.72, 95% CI -1.85 to 0.41). Treatment success and pain frequency were not reported. One study compared web-based education (n=30) with a book-based education (n=30) in IBD patients. The evidence is very uncertain about the effect of this treatment on pain intensity (MD -0.13, 95% CI -1.25 to 0.99). Treatment success and pain frequency were not reported. One study compared yoga (n=50) with no treatment (n=50) in IBD patients. The data reported on treatment success were unclear. Pain frequency and intensity were not reported. One study compared transcranial direct current stimulation (n = 10) to sham stimulation (n = 10) in IBD patients. There may be an improvement in pain intensity when transcranial direct current is compared to sham stimulation (MD -1.65, 95% CI -3.29 to -0.01, low-certainty evidence). Treatment success and pain frequency were not reported. One study compared a kefir diet (Lactobacillus bacteria) to no intervention in IBD patients and provided separate data for their CD participants. The evidence is very uncertain about the effect of this treatment on pain intensity in IBD (MD 0.62, 95% CI 0.17 to 1.07) and CD (MD -1.10, 95% CI -1.67 to -0.53). Treatment success and pain frequency were not reported. Reporting of our secondary outcomes was inconsistent. The most adverse events were reported in the enteric-release glyceryl trinitrate and olorinab studies. In the enteric-release glyceryl trinitrate study, the adverse events were higher in the intervention arm. In the olorinab study, more adverse events were observed in the higher dose arm of the intervention. In the studies on non-drug interventions, adverse events tended to be very low or zero. However, no clear judgements regarding adverse events can be drawn for any interventions due to the low number of events. Anxiety and depression were measured and reported at the end of intervention in only one study; therefore, no meaningful conclusions can be drawn for this outcome.
Authors' conclusions: We found low certainty evidence that transcranial direct current stimulation may improve pain intensity compared to sham stimulation. We could not reach any conclusions on the efficacy of any other interventions on pain intensity, pain frequency, and treatment success. The certainty of the evidence was very low due to the low numbers of studies and participants in each comparison and clinical heterogeneity amongst the studies. While no serious or total adverse events were elicited explicitly with any of the treatments studied, the reported events were very low. The certainty of the evidence for all comparisons was very low, so no conclusions can be drawn.
Copyright © 2021 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
VS: None.
M Gordon: Since August 2016, I have received travel fees to attend international scientific and training meetings from pharma companies. These grants included no honoraria, inducement, advisory role, or any other relationship and were restricted to the travel‐ and meeting‐related costs of attending such meetings. These include: DDW May 2017, World Congress of Gastroenterology October 2017, DDW May 2018, Advances in IBD December 2018, and DDW May 2019. The companies include: Biogaia (2017‐19), Ferring (2018), Allergan (2017), Synergy (bankrupt ‐ 2018), and Tillots (2017‐19). None of these companies has had any involvement in any works completed by me, and I have never received payment for any other activities from them. From August 2019 onwards, I have made a personal undertaking to receive no further funds from any pharmaceutical or formula company in any form for travel or other related activities. This is to lift the limitations such funding has on my ability to act as a first and corresponding author on reviews, in line with Cochrane policies on such matters, and is reported in line with these policies. These current declarations will expire over the next three years, and this statement will be updated regularly to reflect this.
AKA: None.
M Gasparetto: Since 2015, I have received travel fees to attend international scientific and training meetings from pharma companies. These grants included no honoraria, inducement, advisory role, or any other relationship and were restricted to the travel‐ and meeting‐related costs of attending such meetings. These include ESPGHAN 2017 and ESPGHAN 2019, when I was sponsored by Nutricia. None of these companies has had any involvement in any works completed by me, and I have never received payment for any other activities from them.
MS: None.
JV: None.
TD: None
Figures

























Update of
References
References to studies included in this review
Bao 2016 {published data only}
-
- Bao C, Wu L, Wu H, Liu H, Zhao J, Zeng X, et al. Active Crohn's disease treated with acupuncture and moxibustion: a randomized controlled trial. Chinese Acupuncture & Moxibustion 2016;36(7):683-8. - PubMed
Berrill 2014 {published data only}
-
- Berrill JW, Sadlier M, Hood K, Green JT. Mindfulness-based therapy for inflammatory bowel disease patients with functional abdominal symptoms or high perceived stress levels. Journal of Crohn's & Colitis 2014;8(9):945-55. - PubMed
Cox 2020 {published data only}
-
- Cox SR, Lindsay JO, Fromentin S, Stagg AJ, McCarthy NE, Galleron N, et al. Effects of low FODMAP diet on symptoms, fecal microbiome, and markers of inflammation in patients with quiescent inflammatory bowel disease in a randomized trial. Gastroenterology 2020;158:176-88. - PubMed
-
- ISRCTN17061468. Dietary Interventions in inflammatory bowel disease (IBD). www.isrctn.com/ISRCTN17061468 (first received 20 January 2016).
Espi Lopez 2018 {published data only}
Garcia‐Vega 2004 {published data only}
-
- Garcia-Vega E, Fernandez-Rodriguez C. A stress management programme for Crohn's disease. Behaviour Research and Therapy 2004;42(4):367-83. - PubMed
Hawkes 2001 {published data only}
-
- Hawkes ND, Richardson C, Ch'Ng CL, Green JT, Evans BK, Williams J. Enteric-release glyceryl trinitrate in active Crohn's disease: a randomized, double-blind, placebo-controlled trial. Alimentary Pharmacology & Therapeutics 2001;15(12):1867-73. - PubMed
Higgins 2019 {published data only}
-
- Higgins P, Ginsburg D, Gilder K, Gilder K, Walsh B, English B, et al. Safety and efficacy of olorinab, a peripherally restricted, highly-selective, cannabinoid receptor 2 agonist in a phase 2A study in chronic abdominal pain associated with Crohn's disease. Journal of Crohn's and Colitis 2019;13:S318. - PMC - PubMed
-
- Lindstrom B, Ruckle J, Turner S, Stauber K, Brierley SM, English BA. Olorinab, a peripherally restricted, highly selective agonist of the cannabinoid receptor type 2 for the management of visceral pain in inflammatory bowel disease (IBD) - preclinical and early clinical development. Inflammatory Bowel Diseases 2019;25 Suppl 1:S318.
-
- Yacyshyn B, Ginsberg DC, Gilder K, Walsh B, English B, Turner SA, et al. Safety and efficacy of olorinab, a peripherally restricted, highly selective, cannabinoid receptor 2 agonist in a phase 2A study in chronic abdominal pain associated with Crohn's disease. Gastroenterology 2019;156(6):S665.
Mizrahi 2012 {published data only}
-
- Mizrahi MC, Reicher-Atir R, Levy S, Haramati S, Wengrower D, Israeli E, et al. Effects of guided imagery with relaxation training on anxiety and quality of life among patients with inflammatory bowel disease. Psychology & Health 2012;27(12):1463-79. - PubMed
Ozgursoy Uran 2019 {published data only}
-
- Ozgursoy Uran BN, Yildirim Y, Senuzun Aykar F, Unsal B. The effect of web-based education on disease activity, symptom management, and quality of life on patients with inflammatory bowel disease. Journal of Crohn's and Colitis 2018;12(Suppl 1):S582.
-
- Ozgursoy Uran BN, Yildirim Y, Senuzun Aykar F, Unsal B. The effect of web-based education on disease activity, symptom management and quality of life in patients with inflammatory bowel disease: randomized-controlled study. Medical Science 2019;23(98):418-31.
Sharma 2015 {published data only}
-
- Sharma P, Poojary G, Dwivedi SN, Deepak KK. Effect of yoga-based intervention in patients with inflammatory bowel disease. International Journal of Yoga Therapy 2015;25(1):101-12. - PubMed
Tapete 2018 {published data only}
-
- Tapete G, De Bortoli N, Ceccarelli L, Mumolo MG, Vinci E, Albano E, et al. Low-FODMAPs diet improves intestinal symptoms in IBD patients with disease remission: randomized case-control study. Digestive and Liver Disease 2018;50(2):e195.
Tapete 2019 {published data only}
-
- Tapete G, Ceccarelli L, De Bortoli N, Bertani L, Albano E, Baiano Svizzero G. Low- versus high- FODMAPs diet in controlling residual abdominal symptoms in inflammatory bowel disease patients: a randomised controlled study with crossing over. Digestive and Liver Disease 2019;51:e219.
Volz 2016 {published data only}
-
- DRKS00005852. Transcranial direct current stimulation to reduce chronic pain in patients with chronic inflammatory bowel diseases. www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DR... (first received 18 December 2015).
-
- NCT02048137. Transcranial direct current stimulation to reduce chronic pain in patients with chronic inflammatory bowel diseases (tDCS in IBD). clinicaltrials.gov/ct2/show/NCT02048137 (first received 29 January 2014).
-
- Pruess MS, Farmer A, Siegmund B. Inflammatory bowel disease-induced chronic abdominal pain can be ameliorated by transcranial direct current stimulation. Journal of Crohn's and Colitis 2016;10:S289-90.
-
- Volz MS, Farmer A, Siegmund B. Reduction of chronic abdominal pain in patients with inflammatory bowel disease through transcranial direct current stimulation: a randomized controlled trial. Pain 2016;157(2):429-37. - PubMed
References to studies excluded from this review
ACTRN12617000876392 {published data only}
-
- ACTRN12617000876392. IBD mindfulness - mindfulness for youth with Inflammatory Bowel Disease (IBD) and depression trial [A pilot randomised-controlled trial (RCT) of mindfulness-based cognitive therapy (MBCT) for youth with Inflammatory Bowel Disease (IBD) and Depression]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=373115&isRe... (first received 12 June 2017).
Engel 2016 {published data only}
-
- Engel MA, Stracke B. Improvement of health-related quality of life in patients with chronic inflammatory bowel disease after 4 weeks of additive treatment with menthacarin - a randomized, placebo-controlled trial. United European Gastroenterology Journal 2016;4(5):A618.
Forbes 2019 {published data only}
-
- Forbes L. Online Mindfulness Intervention for Inflammatory Bowel Disease Patients: Adherence and Efficacy. Charlotte (NC): The University of North Carolina, 2018.
Gearry 2009 {published data only}
-
- Gearry RB, Irving PM, Barrett JS, Nathan DM, Shepherd SJ, Gibson PR. Reduction of dietary poorly absorbed short-chain carbohydrates (FODMAPs) improves abdominal symptoms in patients with inflammatory bowel disease - a pilot study. Journal of Crohn's & Colitis 2009;3(1):8-14. - PubMed
ISRCTN98226923 {published data only}
-
- ISRCTN98226923. Fermentable dietary carbohydrates as triggers of functional gut symptoms in patients with inflammatory bowel disease. www.isrctn.com/ISRCTN98226923 (first received 20 January 2014).
McCormick 2010 {published data only}
-
- McCormick M, Reed-Knight B, Lewis JD, Gold BD, Blount RL. Coping skills for reducing pain and somatic symptoms in adolescents with IBD. Inflammatory Bowel Diseases 2010;16(12):2148-57. - PubMed
Spagnuolo 2017 {published data only}
-
- Spagnuolo R, Cosco C, Mancina RM, Ruggiero G, Garieri P, Cosco V, et al. Beta-glucan, inositol and digestive enzymes improve quality of life of patients with inflammatory bowel disease and irritable bowel syndrome. European Review for Medical and Pharmacological Sciences 2017;21(Suppl 2):102-7. - PubMed
-
- Spagnuolo R, Ruggiero G, Cosco C, Cosco V, Garieri P, Gidaro A, et al. Beta-glucan, inositol and digestive enzymes in patients with inflammatory bowel disease associated with irritable bowel syndrome. Digestive and Liver Disease 2016;48 (Suppl):e162. - PubMed
Tripp 2017 {published data only}
-
- Tripp DA, Verreault P, Gates J, Ropeleski M, Beyak M. Development and piloting of a cognitive-behavioral self management program for patients with inflammatory bowel disease: an early stage update. Gastroenterology 2017;152(5):S799.
References to studies awaiting assessment
Bao 2021 {published data only}
-
- Bao CH, Zhong J, Liu HR, Gu YP, Wu P, Gu K, et al. Effect of acupuncture-moxibustion on negative emotions and plasma tryptophan metabolism in patients with Crohn's disease at active stage. Zhongguo Zhen Jiu 2021;41(1):17-22. - PubMed
IRCT20120415009475N5 {published data only}
-
- IRCT20120415009475N5. Evaluation of the effects of lyophilized Saccharomyces Boulardii capsules on quality of life and clinical outcomes in children with inflammatory bowel disease (IBD). www.irct.ir/trial/32179 (first received 10 October 2018).
IRCT20200219046553N1 {published data only}
-
- IRCT20200219046553N1. Effect of mindfulness-based cognitive therapy on quality of Life , emotional symptom , dimensions of pain, dispositional mindfulness and disease activity in patients with inflammatory bowel disease. https://en.irct.ir/trial/46526 (registration date 3 April 2020).
JPRN‐UMIN000012635 {published data only}
-
- JPRN-UMIN000012635. Exploratory study evaluating efficacy, safety and mechanism of Daikenchuto on abdominal pain or bloating accompanied by Crohn disease. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000014778 (first received 24 December 2013).
Lee 2021 {published data only}
-
- Lee A, Moulton D, Acra S, Walker L, Mckernan L, Russell A. P039 Clinical hypnosis in pediatric Crohn's Disease: a randomized controlled trial. Gastroenterology 2020;158(3):S100-1. - PubMed
-
- Lee A, Moulton D, Mckernan L, Russell A, Slaughter JC, Acra S et al. Clinical Hypnosis in Pediatric Crohn's Disease: A Randomized Controlled Pilot Study. Journal of pediatric gastroenterology and nutrition 2021;72(3):e63-e70. - PubMed
-
- Lee AD, Moulton DE, Mckernan LC, Russell A, Acra S, et al. 1165 Clinical hypnosis in pediatric Crohn's Disease: a randomised controlled trial. Gastroenterology 158;6:S236. - PubMed
Leiby 2014 {published data only}
-
- Leiby A, Langseder A, Bustami R, Vazirani M, Marchell M, Ferrara M, et al. A randomized, controlled trial of yoga in pediatric inflammatory bowel disease: preliminary findings. American Journal of Gastroenterology 2014;109:S594.
NCT00940576 {published data only}
-
- NCT00940576. Dietetic efficacy of mare's milk for patients with chronic inflammatory bowel diseases. clinicaltrials.gov/ct2/show/NCT00940576 (first received 16 July 2009).
NCT02559037 {published data only}
-
- NCT02559037. Acupuncture treatment for active Crohn's disease. clinicaltrials.gov/ct2/show/NCT02559037 (first received 24 September 2015).
NCT02649075 {published data only}
-
- NCT02649075. To evaluate SBI in the dietary management of mild to moderate Crohn's disease. clinicaltrials.gov/ct2/show/NCT02649075 (first received 4 October 2016).
NCT02963246 {published data only}
-
- NCT02963246. Mindfulness therapy in inflammatory bowel disease (mindfulness). clinicaltrials.gov/ct2/show/NCT02963246 (first received 15 November 2016).
NCT02974322 {published data only}
-
- NCT02974322. A study of efficacy and safety of Mongersen (GED-0301) for the treatment of adult and adolescent subjects with active Crohn's disease. clinicaltrials.gov/ct2/show/NCT02974322 (first received 28 November 2016).
NCT03155945 {published data only}
-
- NCT03155945. Tolerability, pharmacokinetics, and efficacy of APD371 in subjects with Crohn's disease experiencing abdominal pain. clinicaltrials.gov/ct2/show/NCT03155945 (first received 16 May 2017).
NCT03467620 {published data only}
-
- NCT03467620. Cannabidiol usage as an adjunct therapy for Crohn's disease. clinicaltrials.gov/ct2/show/NCT03467620 (first received 16 March 2018).
Neeb 2019 {published data only}
-
- NCT02433470. Association of functional changes in the brain and the perception of pain in patients with chronic inflammatory bowel diseases (IBD). clinicaltrials.gov/ct2/show/NCT02433470 (first received 5 May 2015).
-
- Neeb L, Bayer A, Bayer KE, Farmer A, Fiebach JB, Siegmund B, et al. Transcranial direct current stimulation in inflammatory bowel disease patients modifies resting-state functional connectivity: a RCT. Brain Stimulation 2019;12:978-80. - PubMed
NTR3414 {published data only}
-
- NTR3414. Hypnotherapy for IBD patients with IBS-like symptoms [Hypnotherapy for adolescents and adults with Inflammatory bowel disease and symptoms compatible with Irritable bowel syndrome: a randomized controlled trial]. www.trialregister.nl/trial/3261 (first received 2 May 2012).
Schoultz 2013 {published data only}
-
- ISRCTN27934462. Can mindfulness-based cognitive therapy (a group self-help program) improve the quality of life for inflammatory bowel disease patients? www.isrctn.com/ISRCTN27934462 (first received 2 August 2013).
Schoultz 2015 {published data only}
-
- ISRCTN27934462. Can mindfulness-based cognitive therapy (a group self-help program) improve the quality of life for inflammatory bowel disease patients? www.isrctn.com/ISRCTN27934462 (first received 2 August 2013).
Takakura 2020 {published data only}
-
- Takakura W, Berry SK, Patel D, Govalan R, Ghafari A, Bresee C, et al. Opiate Use in Hospitalized Patients With IBD Can Be Significantly Reduced Using a Proactive Pain Protocol: Results of a Randomized Controlled Trial. Official journal of the American College of Gastroenterology 2020;115:S403.
References to ongoing studies
ISRCTN71618461 {published data only}
-
- ISRCTN71618461. A supported online self-management for symptoms of fatigue, pain and urgency/incontinence in people with inflammatory bowel disease: the IBD-BOOST trial. https://www.isrctn.com/ISRCTN71618461?q=71618461&filters=&sort=&... (first received 2 September 2019). [DOI: 10.1186/ISRCTN71618461] - DOI
NCT03301311 {published data only}
-
- NCT03301311. Personalized research on diet in ulcerative colitis and Crohn's disease (PRODUCE). clinicaltrials.gov/ct2/show/NCT03301311 (first received 4 October 2017).
NCT03348852 {published data only}
-
- NCT03348852. Association between functional changes in the brain and the perception of pain in patients with inflammatory bowel diseases (IBD) - measured with functional magnetic resonance imaging. clinicaltrials.gov/ct2/show/NCT03348852 (first received 21 November 2017).
NCT03422861 {published data only}
-
- NCT03422861. Nabilone use for acute pain in inflammatory bowel disease patients. clinicaltrials.gov/ct2/show/NCT03422861 (first received 6 February 2018).
NCT03667586 {published data only}
-
- NCT03667586. Group cognitive behavioral therapy for IBD patients. clinicaltrials.gov/ct2/show/NCT03667586 (first received 12 September 2018).
NCT03798405 {published data only}
-
- NCT03798405. Reactive vs. proactive pain control in IBD (PAIN-Sparing). clinicaltrials.gov/ct2/show/NCT03798405 (first received 10 January 2019).
NCT03809195 {published data only}
-
- NCT03809195. Clinical hypnosis in pediatric Crohn's disease (HypnoCrohns). clinicaltrials.gov/ct2/show/NCT03809195 (first received 18 January 2019).
NCT03825900 {published data only}
-
- NCT03825900. Transcranial direct current stimulation and the interaction between chronic pain and the intestinal epithelial barrier in patients with chronic inflammatory bowel diseases (IBD). clinicaltrials.gov/ct2/show/NCT03825900 (first received 1 February 2019).
NCT04173273 {published data only}
-
- NCT04173273. A study evaluating the efficacy and safety of etrasimod in the treatment of patients with moderately to severely active Crohn's disease (CULTIVATE). clinicaltrials.gov/ct2/show/NCT04173273 (first received 21 November 2019).
Additional references
AGA 2020
-
- American Gastroenterological Association. American Gastroenterological Association Institute guideline on the use of thiopurines, methotrexate, and anti–TNF-α biologic drugs for the induction and maintenance of remission in inflammatory Crohn's disease. www.gastrojournal.org/article/S0016-5085(13)01521-7/fulltext (accessed 27 April 2020).
Alatab 2020
-
- Alatab S, Sepanlou SG, Ikuta K, Vahedi H, Bisignano C, Safiri S, et al. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterology & Hepatology 2020;5(1):17-30. - PMC - PubMed
Bielefeldt 2009
BSG 2019
-
- British Society of Gastroenterology. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. www.bsg.org.uk/wp-content/uploads/2019/12/BSG-IBD-Guidelines-2019.pdf (accessed 27 April 2019).
Cohen 1988
-
- Cohen J. Statistical Power Analysis in the Behavioral Sciences. 2nd edition. Hillsdale (NJ): Lawrence Erlbaum Associates, Inc, 1988.
Docherty 2011
ECCO 2010
ECCO 2020
-
- European Crohn’s and Colitis Organisation. ECCO guidelines on therapeutics in Crohn's disease: medical treatment. academic.oup.com/ecco-jcc/article/14/1/4/5620479 (accessed 27 April 2020). - PubMed
Egger 1997
Ewais 2019
-
- Ewais T, Begun J, Kenny M, Rickett K, Hay K, Ajilchi B, et al. A systematic review and meta-analysis of mindfulness based interventions and yoga in inflammatory bowel disease. Journal of Psychosomatic Research 2019;116:44-53. - PubMed
Ghosh 2015
Gjuladin‐Hellon 2019
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 6 December 2020. Hamilton (ON): McMaster University (developed by Evidence Prime), 2020. Available at gradepro.org.
Greenley 2013
Halpin 2012
-
- Halpin SJ, Ford AC. Prevalence of symptoms meeting criteria for irritable bowel syndrome in inflammatory bowel disease: systematic review and meta-analysis. American Journal of Gastroenterology 2012;107(10):1474-82. - PubMed
Higgins 2021
-
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2. Cochrane, 2021.
Iheozor‐Ejiofor 2019
-
- Iheozor-Ejiofor Z, Gordon M, Clegg A, Freeman SC, Gjuladin-Hellon T, MacDonald JK, et al. Interventions for maintenance of surgically induced remission in Crohn’s disease: a network meta-analysis. Cochrane Database of Systematic Reviews 2019, Issue 9. Art. No: CD013210. [DOI: 10.1002/14651858.CD013210.pub2] - DOI - PMC - PubMed
Kafil 2018
Kaplan 2015
-
- Kaplan G. The global burden of IBD: from 2015 to 2025. Nature Reviews. Gastroenterology & Hepatology 2015;12:720-7. - PubMed
Limketkai 2019
-
- Limketkai BN, Iheozor-Ejiofor Z, Gjuladin-Hellon T, Parian A, Matarese LE, Bracewell K, et al. Dietary interventions for induction and maintenance of remission in inflammatory bowel disease. Cochrane Database of Systematic Reviews 2019, Issue 2. Art. No: CD012839. [DOI: 10.1002/14651858.CD012839.pub2] - DOI - PMC - PubMed
Lin 2018
Long 2016
NICE 2019
-
- National Institute for Health and Care Excellence. Crohn's disease: management. www.nice.org.uk/guidance/ng129 (accessed 1 July 2019).
Norton 2017
-
- Norton C, Czuber-Dochan W, Artom M, Sweeney L, Hart A. Systematic review: interventions for abdominal pain management in inflammatory bowel disease. Alimentary Pharmacology & Therapeutics 2017;46:115-25. - PubMed
Odes 2017
Paiotti 2012
-
- Paiotti APR, Marchi P, Miszputen SJ, Oshima CTF, Franco M, Ribeiro DA. The role of nonsteroidal antiinflammatory drugs and cyclooxygenase-2 inhibitors on experimental colitis. In Vivo 2012;26(3):381-93. - PubMed
Pauly 2017
Prince 2016
-
- Prince AC, Myers CE, Joyce T, Irving P, Lomer M, Whelan K. Fermentable carbohydrate restriction (low FODMAP diet) in clinical practice improves functional gastrointestinal symptoms in patients with inflammatory bowel disease. Inflammatory Bowel Diseases 2016;22(5):1129-36. - PubMed
RCP 2012
-
- Royal College of Physicians. Report of the results for the national clinical audit of adult inflammatory bowel disease inpatient care in the UK. https://www.rcplondon.ac.uk › file › download (accessed 5 May 2021).
Review Manager 2020 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Satsangi 2006
Srinath 2012
Thapa 2019
-
- Thapa N, Kappus M, Hurt R, Diamond S. Implications of the opioid epidemic for the clinical gastroenterology practice. Current Gastroenterology Reports 2019;21(9):44. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

