Prognostic characterization of immune molecular subtypes in non-small cell lung cancer to immunotherapy
- PMID: 34844602
- PMCID: PMC8628446
- DOI: 10.1186/s12890-021-01765-3
Prognostic characterization of immune molecular subtypes in non-small cell lung cancer to immunotherapy
Abstract
Background: Non-small cell lung cancer (NSCLC) was usually associated with poor prognosis and invalid therapeutical response to immunotherapy due to biological heterogeneity. It is urgent to screen reliable biomarkers, especially immunotherapy-associated biomarkers, that can predict outcomes of these patients.
Methods: Gene expression profiles of 1026 NSCLC patients were collected from The Cancer Genome Atlas (TCGA) datasets with their corresponding clinical and somatic mutation data. Based on immune infiltration scores, molecular clustering classification was performed to identify immune subtypes in NSCLC. After the functional enrichment analysis of subtypes, hub genes were further screened using univariate Cox, Lasso, and multivariate Cox regression analysis, and the risk score was defined to construct the prognostic model. Other microarray data and corresponding clinical information of 603 NSCLC patients from the GEO datasets were applied to conduct random forest models for the prognosis of NSCLC with 100 runs of cross-validation. Finally, external datasets with immunotherapy and chemotherapy were further applied to explore the significance of risk-scores in clinical immunotherapy response for NSCLC patients.
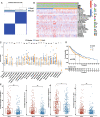
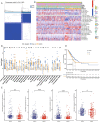
Results: Compared with Subtype-B, the Subtype-A, associated with better outcomes, was characterized by significantly higher stromal and immune scores, T lymphocytes infiltration scores and up-regulation of immunotherapy markers. In addition, we found and validated an eleven -gene signatures for better application of distinguishing high- and low-risk NSCLC patients and predict patients' prognosis and therapeutical response to immunotherapy. Furthermore, combined with other clinical characteristics based on multivariate Cox regression analysis, we successfully constructed and validated a nomogram to effectively predict the survival rate of NSCLC patients. External immunotherapy and chemotherapy cohorts validated the patients with higher risk-scores exhibited significant therapeutic response and clinical benefits.
Conclusion: These results demonstrated the immunological and prognostic heterogeneity within NSCLC and provided a new clinical application in predicting the prognosis and benefits of immunotherapy for the disease.
Keywords: Immune molecular subtype; Immunotherapy; Non-small cell lung cancer; Prognosis; Risk stratification.
© 2021. The Author(s).
Conflict of interest statement
All authors declare that they have no competing interests.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

